
Keratoconus is a progressive corneal disease in which the cornea thins and bulges, forming an irregular cone shape. The corneal thinning occurs due to a breakdown in the collagen matrix that normally gives the cornea its shape and stability. Risk factors for keratoconus are multifactorial, with eye rubbing and genetics the leading contributors. Some ethnicities have higher rates of keratoconus than others.1
AT A GLANCE
- Only epithelium-off CXL is approved for use by the FDA.
- Epithelium-on CXL can be easier on patients, with faster recovery and less risk of infection.
- CXL can be a comanaged procedure if the comanaging doctors are comfortable with the arrangement.
Some mild stage keratoconus patients can be successfully managed by correcting distortions in vision with spectacles or specialty contact lenses. If the cornea becomes too thin or severely scarred, however, such as in the advanced stages of keratoconus, a corneal transplant may be the best treatment. Transplant options now include deep anterior lamellar keratoplasty (DALK) and penetrating keratoplasty (PKP).
TREATING KERATOCONUS WITH CXL
Twenty years ago, corneal collagen crosslinking (CXL) techniques were developed by researchers at the University of Dresden.2 We’ve seen the benefits of CXL in strengthening the cornea, flattening keratometric values, and improving UCVA and BCVA. In 2016, the FDA approved the KXL System and Photrexa riboflavin formulations (Avedro; acquired by Glaukos in 2019) for performing epithelium-off (epi-off) CXL procedures.
We recommend CXL to patients as early as possible in the progression of keratoconus (Figure 1). By halting the progression of keratoconus early, we give patients their best chance for maintaining the best visual potential. In our practice we have treated patients as young as 12 years and successfully halted the progression of keratoconus before it affected their vision. We expect the likelihood of future corneal transplants to be greatly reduced for patients with keratoconus due to early intervention with CXL.
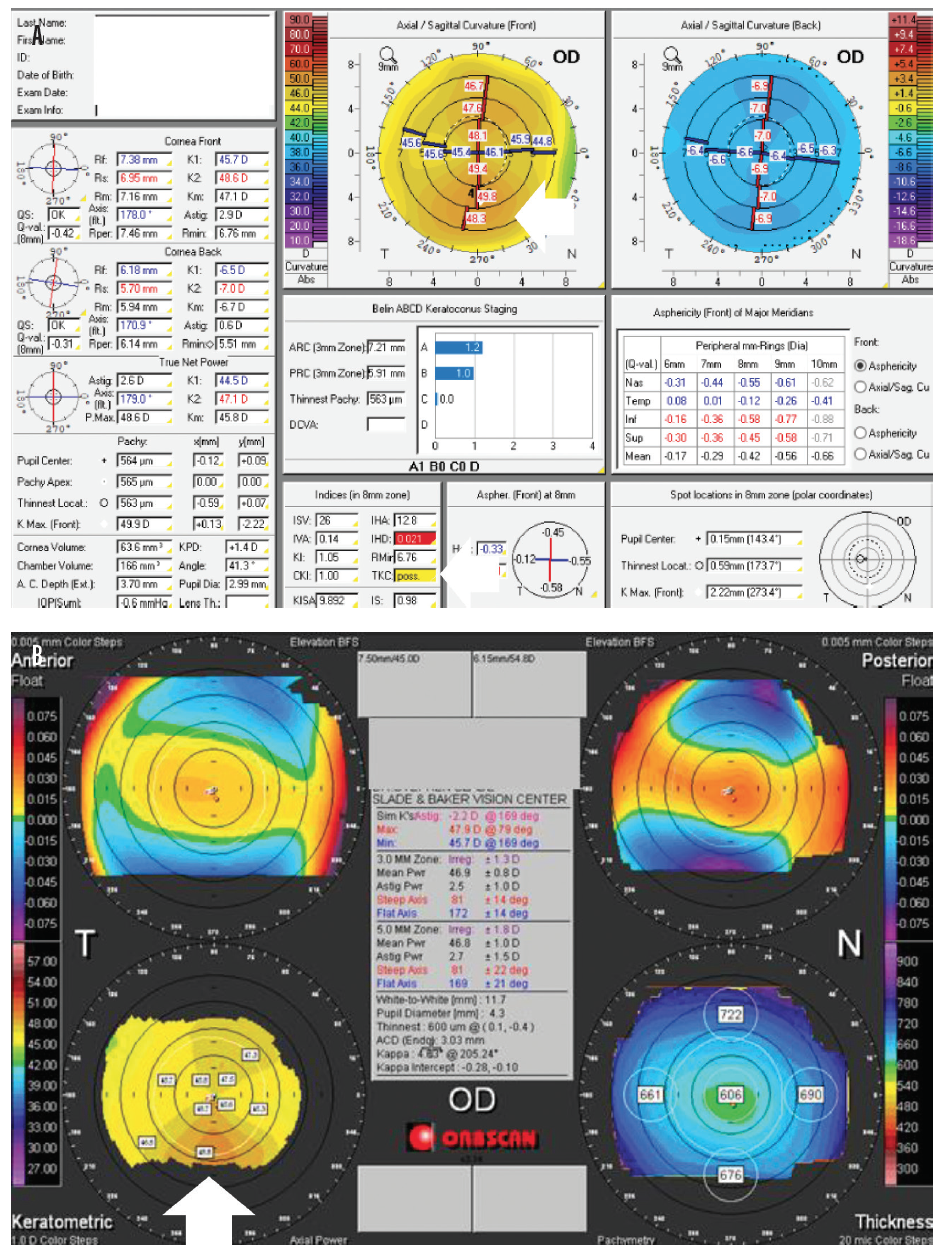
Figure 1. Pentacam (Oculus Optikgeräte) images showing early keratoconus. Topographical keratoconus classification is possible (A). Notice the inferior steepening in early keratoconus (B).
The benefits of CXL are not limited to keratoconus patients. Improvements in corneal stability have been documented in patients with pellucid marginal degeneration (PMD; Figure 2), corneal ectasia after refractive surgery, and vision fluctuations after radial keratotomy.3
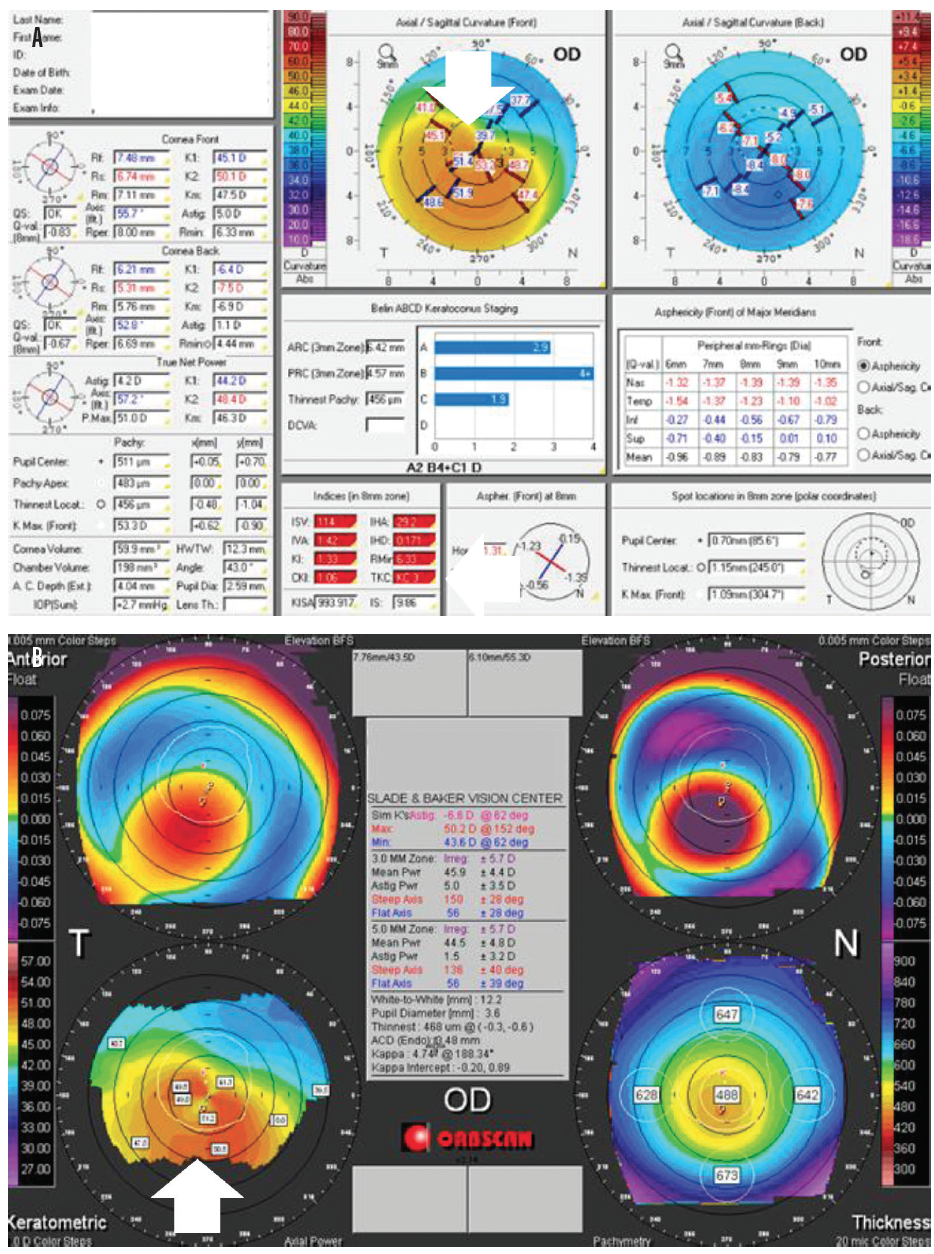
Figure 2. Pentacam images showing pellucid marginal degeneration. Topographical keratoconus classification (TKC) value (in red, bottom of center column) = KC3 (A). TKC gives a grading from 0 (normal) to 4 (severe). This grading scale is helpful when classifying a new keratoconus patient or monitoring an existing patient. Notice the crab-claw appearance indicating pellucid marginal degeneration in the keratometric images (B).
EPI-ON VS EPI-OFF
Is it better to leave the epithelium intact or remove it for CXL? The only FDA approved device for CXL is labeled for epi-off CXL. Although removing the epithelium allows faster penetration of riboflavin into the corneal stroma, in our practice we have seen that it may also result in longer healing time (especially in a diseased cornea), an increase in postoperative pain, and higher risks of scarring and infection.
In our experience, the epithelium-on (epi-on) procedure allows patients to experience faster, more comfortable recovery while also reducing the risk of infection and scarring. Epi-on CXL requires a slightly different riboflavin solution to penetrate the intact epithelium.
Slade & Baker Vision, where I practice, is involved in clinical trials investigating the use of supplemental oxygen to further improve the efficacy of epi-on CXL. This trial protocol could limit the risk of endothelial damage in corneas thinner than 400 µm. Earlier versions of epi-on CXL were not as effective as epi-off because riboflavin solutions and oxygenation levels were not optimized.
Because the current FDA-approved method is epi-off, virtually no insurance covers epi-on CXL, despite its potential advantages. Meanwhile, based on personal correspondence with our colleagues in Europe, where CXL was developed, epi-on CXL is overwhelmingly preferred there.
Our practice has participated in some of the largest FDA clinical trials for both epi-on and epi-off CXL. A US phase 3 trial of epi-on CXL has completed enrollment, and results are expected to be announced this year.4,5
WHEN TO REFER
For optometrists, the question of when to refer a patient with keratoconus for treatment is crucial. Age is an important consideration. If the patient is younger than 25 years, we suggest initiating treatment as soon as the diagnosis of keratoconus is made. If we wait for progression, we risk limiting the patient’s potential for the best quality of vision. If the patient is older than 25 and has signs of increasing irregular astigmatism and/or distorted topographies, we refer him or her for a CXL surgical evaluation and treatment.
Regardless of age and corneal stability, if a patient has been diagnosed with keratoconus he or she must be educated regarding treatment with CXL. Patients should be aware of the benefits of CXL, the possibility of progression in the future, and the ways that progression can affect BCVA. The Pentacam (Oculus Optikgeräte) corneal topographer is a great diagnostic tool for monitoring corneal curvature, evaluating for ectasia, and grading stage of keratoconus.
Understanding the patient’s family history can also help with a referral decision. If other family members have been diagnosed with keratoconus or have had a corneal transplant, the patient is more likely to have keratoconus, and an appropriate referral should be considered promptly.
In addition, pertinent information about eye-rubbing should be gathered. If the patient admits to eye-rubbing, educate him or her that the cornea can be further weakened and counsel discontinuing the habit immediately. In our experience, eye-rubbing can also be an underlying symptom of dry eyes or allergies, and these conditions should be treated concurrently if present.
COMANAGEMENT OF CXL
CXL can be a comanaged procedure if the patient chooses and the comanaging doctor is comfortable with the arrangement. Most patients return to their comanaging doctor once the condition is stable. We recommend fitting for a scleral lens, if needed, as early as 1 month after CXL or when the cornea is generally stable. There is some evidence that post-CXL corneas can continue normalizing and flattening in small degrees over years.
Patients can wear their spectacles immediately after the treatment and return to their scleral or soft contact lenses 48 hours after CXL or when the epithelium is healed. If the patient is a rigid gas permeable (RGP) lens wearer, we typically wait 2 weeks after CXL to resume RGP wear to limit epithelial disruption.
Patients will be using eye drops after treatment and may have a bandage contact lens placed for comfort for the first few days. We advise removing the bandage lens once the epithelium is healed. First, the lens is loosened with a generous saline rinse and is then gently removed with forceps.
Common patient symptoms after CXL are light sensitivity and tenderness for the first few days. Visual and refractive fluctuations can be common for up to 6 months. Most patients will be able to return to routine activities such as driving and working within a few days. Always let patients know when they are legally cleared to drive, and always remind them not to rub their eyes.
WORKING TOGETHER
Slade & Baker Vision has relied on comanagement for more than 30 years, and we place high importance on communicating with our referring doctors. We also emphasize to patients the importance of long-term optometric care before and after CXL. When optometry and ophthalmology practices work together, patients benefit from our combined areas of expertise.
We highly recommend that optometrists build a comanagement relationship with an ophthalmologist they trust, even if the surgeon is hours away from one’s practice. Your patients deserve the best, and with CXL we have better treatment options than ever before. When optometrists catch keratoconus in its early stages, we have an opportunity to prevent permanent vision changes and help our patients safely achieve their best vision.
1. Pearson AR, Soneji B, Sarvananthan N, Sandford-Smith JH. Does ethnic origin influence the incidence or severity of keratoconus? Eye (Lond). 2000;14:625-628.
2. Wollensak G, Spoerl E, Seiler T. Riboflavin/ultraviolet-a-induced collagen crosslinking for the treatment of keratoconus. Am J Ophthalmol. 2003;135(5):620-627.
3. Galvis V, Tello A, Ortiz AI, Escaf LC. Patient selection for corneal collagen cross-linking: an updated review. Clin Ophthalmol. 2017;11:657-668.
4. Avedro completes enrollment in pivotal U.S. phase 3 epi-on corneal cross-linking clinical trial for progressive keratoconus [press release]. May 1, 2019. Waltham, MA: Avedro. bit.ly/MODnews0320no4. Accessed January 30, 2020.
5. Study to evaluate the safety and efficacy of epi-on corneal cross-linking in eyes with progressive keratoconus. NCT03442751. clinicaltrials.gov. Accessed January 30, 2020.

