Dry eye disease (DED) occurs when the quantity and/or quality of tears fails to keep the surface of the eye adequately lubricated. Experts estimate that dry eye affects millions of adults in the United States.1 In today’s ophthalmic landscape, it is imperative that optometrists and ophthalmologists continue to study the underlying etiologies together, while engaging in a system of collaborative, point-of-care testing to maximize professional strengths, share information, and move toward better patient outcomes.
Developing a strong network of communication and referrals between eye care professionals is key to successful patient care. Comanagement starts with educating patients when they first present with a chronic condition. At that point, it is understood that they may need to see more than one eye care professional. This is the time to engage patients in order to optimize the surgical intervention, educate patients about their condition, and introduce a long-term maintenance regimen.
Preoperative Dry Eye Disease Protocols
Collectively in our respective practices, many patients we see may not be symptomatic, but are pre-symptomatic. We see patients with cataracts who are not ready for surgery but will be in a year or two. We can start educating those patients and preparing them before and after surgery, including the potential impact of DED on their surgical outcome. Ideally, when we are able to educate patients early, there is much better management pre- and postoperatively, as well as higher patient satisfaction.
From a surgical perspective, patients may only be seen once when they come in for a surgical evaluation, which includes preoperative measurements for cataract surgery and critical tests for dry eye. For cases like this, we use a protocol algorithm created by the American Society of Cataract and Refractive Surgery (ASCRS).2 The protocol begins with a subjective questionnaire that is very helpful for identifying symptoms indicative of dry eye disease in our surgical patients. Next, we conduct some objective testing, such as osmolarity testing, using the TearLab Osmolarity System (Figure 1). We conduct MMP-9 testing, which is also outlined in the ASCRS protocol. Both of these tests are deemed “essential” preoperative diagnostic tests by the ASCRS.2 If any one of the tests are abnormal, that triggers the next stage of observation as we become suspicious that the patient has some form of ocular surface disease.
Figure 1. TearLab Osmolarity System.
Let’s say that, at this point, we do not know what causes the abnormal test result. It could be an allergy or blepharitis, but it certainly could be dry eye, particularly if the tear osmolarity is abnormal. So, we’re now going to examine the patient and try to determine if this is a visually or nonvisually significant ocular surface disease. In other words, is it going to affect patients’ vision and affect biometry? If the answer is yes, we need to delay surgery, initiate a treatment, and remeasure.
For example, if a minimally symptomatic patient comes in presenting with slight inferior staining and an osmolarity score of 330 mOsm/L, we would consider that more than a standard deviation from the norm, indicating the patient may have a problem with his or her tear homeostasis. In general, if a patient’s inter-eye difference is 8 mOsm/L or greater, or osmolarity are over 308 mOsm/L, then it is time to investigate further before doing anything else.
Essentials of Diagnosing Dry Eye Disease
Point-of-care diagnostic testing has led to a paradigm shift toward standard of care. If DED is suspected, we use tear film osmolarity, MMP-9, and traditional tests to identify staining, such as fluorescein or lissamine green. Additionally, every patient gets a validated questionnaire.
It is important not to minimize the clinical exam. Equally important are point-of-care tests that include a dynamic, comprehensive exam to look for staining; evaluation of the meibomian glands with meibography (if available); topography measurements to detect any hot or cool spots or asymmetry; and testing for ocular scatter index. This serves not only to provide the best treatment strategy and allow for better decision-making but also to provide a level of confidence when sharing results and educating the patient.
Patients appreciate numbers and tear osmolarity provides a quantitative metric that you can interpret for them. Rather than providing a diagnosis based solely on insights, you are able to give them data-supported information. This enables a better understanding of the clinical process in order to get the best possible outcome. As physicians, our job is to figure out why they have a problem, set appropriate expectations, and present the best possible treatment plan to manage their disease.
There is no patient ‘type’
Objective tests are utilized in all facets of eyecare. Consider contact lens patients. How are we treating contact lens patients differently knowing that, over time, there is a strong chance of the patient developing meibomian gland dysfunction?
Screening for ocular surface disease is just as important for the glaucoma patient as it is for the retina patient. Conducting objective tasks, such as osmolarity, shouldn’t be defined by the etiology. It is imperative to realize that patients can get ocular surface disease in many different forms, such as toxicity from glaucoma medications.
The Formula for Long-term Monitoring of Dry Eye Disease
The one thing we can all agree on is the need for a point-of-care protocol. It is important to decide which tools you are going to include in that protocol, whether you use osmolarity, MMP-9, or lissamine green testing. This leads to a protocol that is predictable and anticipated, which is important not only for the clinician but the staff as well. If you don’t have a protocol set in place, it makes for an inefficient practice.
For long-term monitoring of DED, osmolarity is a good marker for therapy (Figure 2). For example, patients who were started on cyclosporine could experience the osmolarity drop after a certain amount of time. When the patient was taken off, the osmolarity went back up.3 The same applies with diverse therapies such as Omega-3s4 and compounded hormonal therapies. Osmolarity is a strong biomarker for long-term DED testing and monitoring. It may be that a patient is placed on a treatment plan that doesn’t work. If osmolarity doesn’t fall, it gives us the information and confidence that we need to try a different treatment strategy.
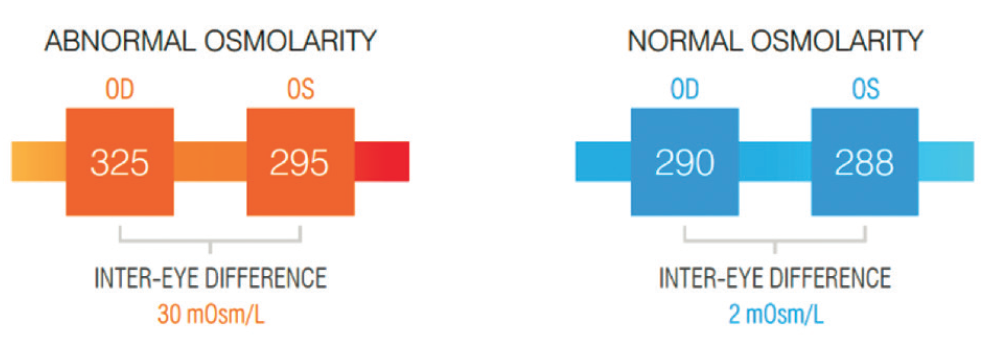
Figure 2. Abnormal versus normal osmolarity biomarkers.
This makes the argument for suggesting alternative treatment plans based on data rather than a gut feeling based on previous patient outcomes. For example, if recommending an amniotic membrane, or punctal occlusion, the patient may have more confidence if you’re able to demonstrate that his or her osmolarity hasn’t fallen with previous treatment plans. He or she may be more reluctant to undergo that regimen. Now you have more objective evidence to show which treatments aren’t working and the confidence to suggest a new treatment strategy.
Conclusion
Point-of-care testing comes down to consistency with a main goal of reestablishing tear homeostasis. There are several tests that monitor different aspects of homeostasis, but many measure aspects of dry eye that have considerable variability of time. Compared to other signs of DED, the least amount of variability is offered with osmolarity,3 which is a strong start for point-of-care testing.
The objective nature of modern point-of-care testing facilitates a good OD-MD relationship. This type of data are not subjective or based on personal opinion, but rather facts around the ocular surface health of a patient. The patient can therefore be seamlessly managed between ODs and MDs, ensuring better preoperative and postoperative care. It is a scenario where all parties benefit.
1. Facts about dry eye. nei.nih.gov. https://nei.nih.gov/health/dryeye/dryeye. Accessed August 19, 2019.
2. Starr CE, Gupta PK, Farid M, et al. An algorithm for the preoperative diagnosis and treatment of ocular surface disorders. J Cataract Refract Surg. 2019;45(5):669-684.
3. Sullivan BD, Crews LA, Sönmez B, et al. Clinical utility of objective tests for dry eye disease: variability over time and implications for clinical trials and disease management. Cornea. 2012;31(9):1000-1008.
4. Ton J, Korownyk C. Omega-3 supplements for dry eye. Can Fam Physician. 2018;64(11):826.





