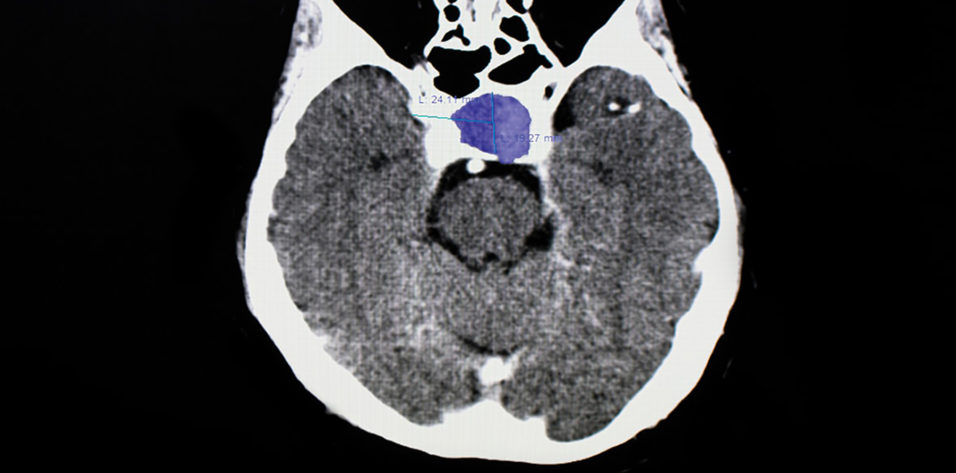
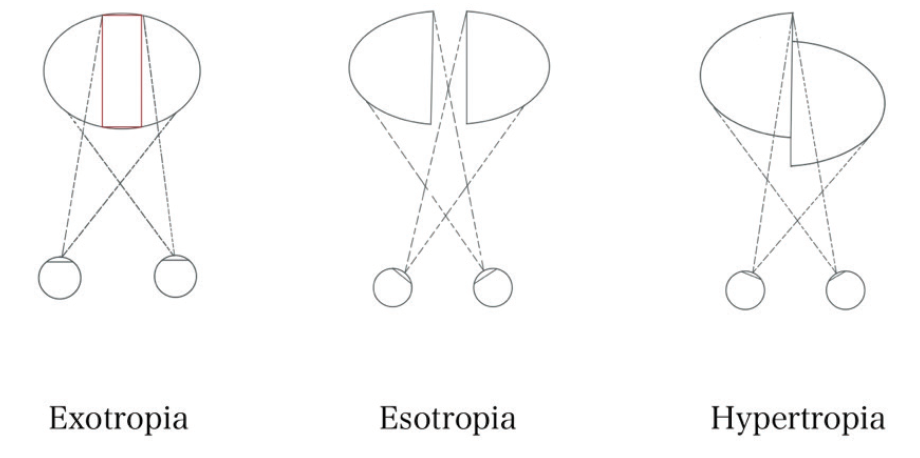
Pituitary macroadenomas are benign tumors. The most common subtype is clinically nonfunctioning pituitary adenoma, which is defined by an absence of clinical evidence of hormonal hypersecretion.1 Patients with pituitary macroadenomas may present to an eye clinic with symptoms related to mass effect on surrounding structures, which can include headache, bitemporal visual defects, and ocular motor deficits (see Key Ocular Findings in Pituitary Macroadenoma).2-4
If you suspect that a patient has a nonfunctioning pituitary adenoma, the following steps are warranted: visual acuity testing, color plates, a cranial nerve examination, an assessment for a relative afferent pupillary defect, an evaluation of the optic discs’ appearance, visual field testing, and OCT imaging. In addition to narrowing the differential diagnosis, the information gathered by these assessments can guide treatment strategies, predict prognostic factors for recovery, inform providers’ and patients’ expectations, and document postoperative recovery after resection or radiotherapy.5
VISUAL FIELD DEFECTS
Visual field testing is central to the workup of pituitary adenomas. In a review of eight studies, visual field disturbances were the most common symptoms at presentation, found in approximately 46% of all patients.4 It is important to note, however, that slow tumor growth may delay patients’ presentation to an eye clinic, and some asymptomatic cases are detected incidentally on glaucoma screening.
Bitemporal hemianopsia can occur as a result of suprasellar growth of the pituitary lesion and direct inferior compression of the optic chiasm first, where the axons that give rise to the superior temporal visual field cross (see Differential Diagnosis of Bitemporal Hemianopsia).6
Tumors may grow asymmetrically, and different configurations of the optic chiasm relative to the pituitary gland can contribute to the observed pattern of presentation. If the optic chiasm sits anterior to the pituitary gland within the tuberculum sellae, referred to as a prefixed chiasm, a pituitary adenoma is more likely to compress the posterior chiasm and optic tracts to produce a macular bitemporal hemianopsia or homonymous visual field loss. Conversely, postfixed chiasms overlying the dorsum sellae may produce patterns of visual field loss related to the optic nerve or a junctional scotoma due to compression at the confluence of the optic nerve and chiasm.6
OCULOMOTOR DEFICITS
Cranial nerve (CN) palsies occur as a result of compression caused by the direct extension of a tumor out of the sella and into the adjacent cavernous sinuses. Multiple palsies of CN III, IV, V1, V2, and/or VI localize to this region. A patient with any of several CN palsies will complain of diplopia and, in severe cases, may present acutely with headache as a result of pituitary apoplexy. Acute-onset diplopia and headache in this scenario should prompt immediate neuroimaging (see Pituitary Apoplexy). Pituitary adenomas may also cause diplopia due to decompensation of a preexisting phoria by the lack of crossover of the remaining visual field in the setting of complete hemianopsia (see Hemifield Slide).
See-saw nystagmus (SSN) is a rare ocular motor syndrome characterized by a cycle of elevation and intorsion of one eye with synchronous depression and extorsion of the other, a scenario that is inverted in the next half-cycle. Two patterns of SSN exist. The pendular-type SSN is more typical of parasellar masses, and it is differentiated from jerk SSN by its smooth and rolling movements.7 Although the underlying pathophysiology of pendular SSN remains a subject of debate, a two-hit hypothesis has been proposed. First, compression of the chiasm may lead to a retinal calibration error of the vestibulo-ocular reflex, causing a disturbance in the roll plane (see Hemifield Slide). Second, compression of the interstitial nucleus of Cajal or the rostral interstitial nucleus of the medial longitudinal fasciculus can lead to nystagmus in the pitch and roll planes.7
OCULAR IMAGING IN DIAGNOSIS AND MANAGEMENT
OCT imaging is useful for visualizing the retinal nerve fiber layer (RNFL) and macular ganglion cell layer (GCL) in patients with visual field loss from chiasmal compression (Figure 1). Pituitary adenomas cause compression of the axons from the nasal retinal fibers, which correspond to the temporal visual fields. This damage is reflected after weeks to months in binasal macular ganglion cell atrophy and its corresponding temporal and then nasal peripapillary RNFL. When the superior and inferior RNFL quadrants are relatively spared, atrophy with a characteristic bow-tie or band appearance eventually occurs.8
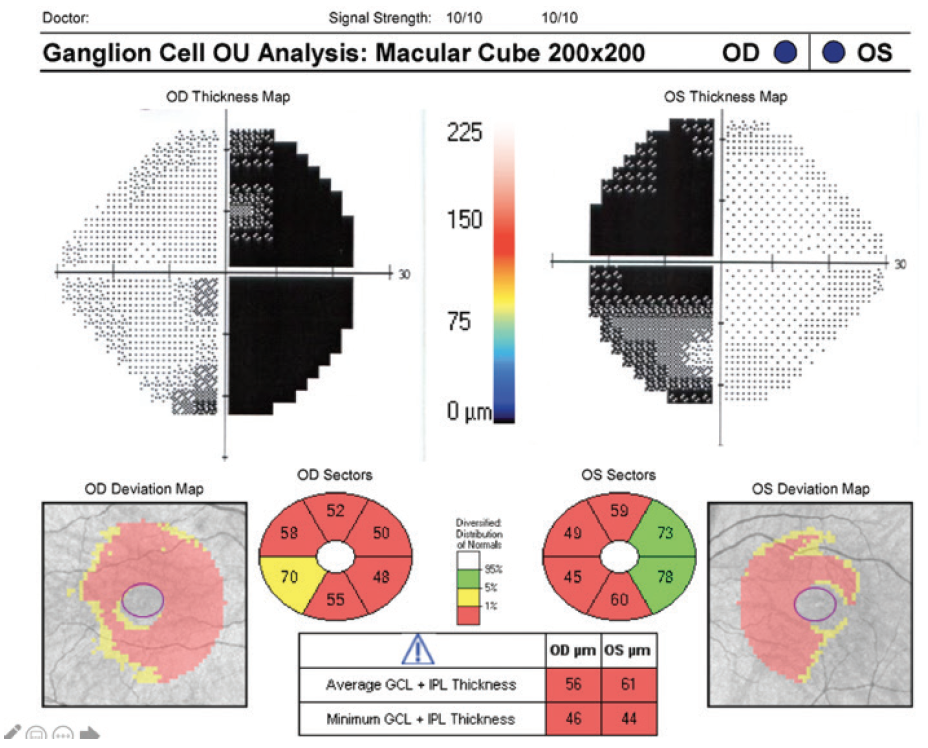
Figure 1. Binasal macular GCL atrophy mirrors bitemporal hemianopsia in pituitary adenoma chiasm compression. Fields are shown overlying their respective eyes on OCT for direct comparison.
Original field and OCT scan courtesy of Randy Kardon, MD, PhD
Recent studies have shown that OCT imaging can provide useful guidance on treatment strategy. Because extensive RNFL thinning and macular GCL thinning indicate retrograde atrophy of fibers from chiasmal compression, patients with intact RNFL and GCL on OCT at diagnosis may have a greater potential for recovering optic nerve function and visual fields after surgical intervention.8
MANAGEMENT
MRI with and without contrast of the brain and/or orbits with attention to the sella is the recommended medium for diagnosis (Figure 2). A patient with a confirmed pituitary macroadenoma should be referred to both neurosurgery and endocrinology. Asymptomatic patients with incidental macroadenomas should receive continued ophthalmologic surveillance because any new visual symptoms strongly indicate a need for surgical intervention. Although there are no established guidelines for the length of ophthalmologic follow-up, lesion size and a progression of visual field defects should inform decisions regarding timeline.
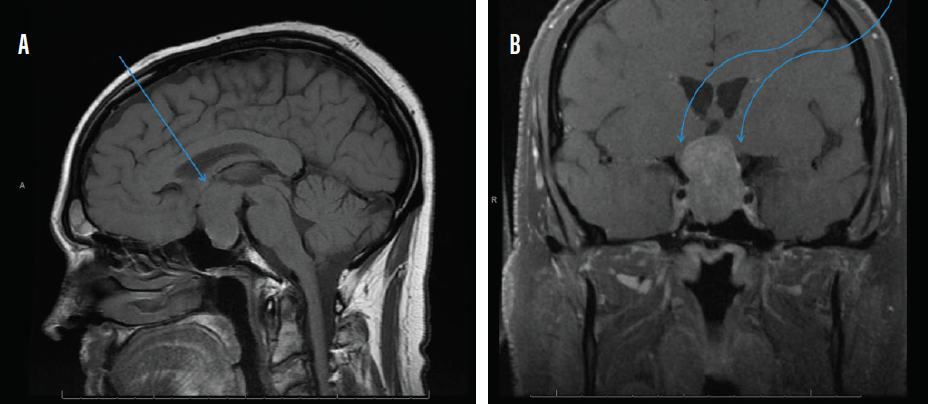
Figure 2. Sagittal midline precontrast T1-weighted MRI showing enlargement of the sella turcica and superior extension of a pituitary macroadenoma (arrow, A). Coronal T1-weighted MRI with contrast showing the optic chiasm stretched and compressed over the mass (arrows, B).
If visual impairment secondary to pituitary macroadenoma is confirmed or progressive, first-line treatment is surgical resection with endoscopic or microscopic transsphenoidal surgery.9 Bromocriptine can be used for prolactin-secreting adenomas (usually small). Large masses may require superior craniotomy approaches, whereas gamma knife radiotherapy is reserved for large tumor remnants and the management of regrowth after surgery.9 In a review of 19 studies that describe visual outcomes after resection, approximately 40% of patients recovered completely, 80% showed an overall improvement, and 2% reported further deterioration in visual function.10 Preoperative visual field assessments are essential to estimate visual outcomes after resection and to set both patients’ and providers’ expectations.
CONCLUSION
By understanding the clinical signs associated with pituitary adenomas, you can ensure that patients with associated visual defects receive an early diagnosis and a thorough preoperative assessment. The information gathered can inform treatment plans and better predict ophthalmologic outcomes after treatment.
1. Ntali G, Wass JA. Epidemiology, clinical presentation and diagnosis of non-functioning pituitary adenomas. Pituitary. 2018;21(2):111-118.
2. Chen L, White WL, Spetzler RF, Xu B. A prospective study of nonfunctioning pituitary adenomas: presentation, management, and clinical outcome. J Neurooncol. 2011;102(1):129-138.
3. Cury MLCD, Fernandes JC, Machado HR, Elias LL, Moreira AC, de Castro M. Non-functioning pituitary adenomas: clinical feature, laboratorial and imaging assessment, therapeutic management and outcome. Arq Bras Endocrinol Metabol. 2009;53(1):31-39.
4. Molitch ME. Nonfunctioning pituitary tumors and pituitary incidentalomas. Endocrinol Metab Clin North Am. 2008;37(1):151-171, xi.
5. Newman SA, Turbin RE, Bodach ME, et al. Congress of neurological surgeons systematic review and evidence-based guideline on pretreatment ophthalmology evaluation in patients withsuspected nonfunctioning pituitary adenomas. Neurosurgery. 2016;79(4):E530-532.
6. Winges KM, Jivraj I. Topical diagnosis of visual field loss. In: Prajna NV, ed. Peyman’s Principles and Practice of Ophthalmology. Jaypee Brothers; 2018:1599-1628.
7. Woo PY, Takemura S, Cheong AM, et al. Pendular seesaw nystagmus in a patient with a giant pituitary macroadenoma: pathophysiology and the role of the accessory optic system. J Neuroophthalmol. 2018;38(1):65-69.
8. Abouaf L, Vighetto A, Lebas M. Neuro-ophthalmologic exploration in non-functioning pituitary adenoma. Ann Endocrinol (Paris). 2015;76(3):210-219.
9. Esposito D, Olsson DS, Ragnarsson O, Buchfelder M, Skoglund T, Johannsson G. Non-functioning pituitary adenomas: indications for pituitary surgery and post-surgical management. Pituitary. 2019;22(4):422-434.
10. Muskens IS, Zamanipoor Najafabadi AH, Briceno V, et al. Visual outcomes after endoscopic endonasal pituitary adenoma resection: a systematic review and meta-analysis. Pituitary. 2017;20(5):539-552.