

Fuchs endothelial corneal dystrophy is the most common indication for keratoplasty in the United States.1 Endothelial keratoplasty (EK) is the current standard of care when the disease causes problematic visual symptoms. Descemet stripping endothelial keratoplasty (DSEK) is the version of EK most frequently performed, but Descemet membrane endothelial keratoplasty (DMEK) has been growing in popularity over the past 10 years due to its lower risk of rejection, faster recovery rate, better visual acuity outcomes, and lower induction of refractive error.2
AT A GLANCE
- Endothelial keratoplasty is the current standard of care when Fuchs endothelial corneal dystrophy causes problematic visual symptoms.
- Descemet stripping only (DSO) is a technique in which dysfunctional central corneal endothelium and Descemet membrane are removed, allowing healthier peripheral cells to migrate into the area.
- DSO uses no donor tissue, so there is no rejection risk and no need for long-term steroid use.
Although both of these EK procedures have a high success rate, the risks associated with donor transplantation are still present. Descemet stripping only (DSO), sometimes also called descemetorhexis without EK (DWEK), is a technique in which dysfunctional central corneal endothelium and Descemet membrane are removed, allowing healthier peripheral cells to migrate into the area.
DSO uses no donor tissue, so there is no rejection risk and no need for long-term steroid use. Demand on the donor tissue pool is also reduced as a result. The adjunctive use of rho kinase (ROCK) inhibitors has been shown to speed the resolution of corneal edema after DSO.3 In the cell cycle, rho kinase halts the progression of corneal endothelial cells. Therefore, ROCK inhibition can lead to proliferation and suppression of apoptosis in corneal endothelial cells.4
CASE PRESENTATION
A 67-year-old White woman with Fuchs endothelial corneal dystrophy was referred from the general optometry clinic for a corneal evaluation. She had noted fluctuating vision and difficulty with glare that had been worsening over the past few months. Her ocular history was remarkable for nuclear sclerotic cataracts, dry eye syndrome, and retinopexy for a retinal tear in the right eye.
On examination, Snellen BCVA was 20/50 OD (-8.75 +2.75 x 076°) and 20/30 OS (-7.25 +1.75 x 085°). Both corneas had a 3+ central guttae plaque and trace anterior stromal haze. The peripheral corneas were clear of guttae. No clinically apparent corneal edema was noted at the slit lamp. The patient also had 1+ nuclear sclerosis in each eye.
Pachymetry measured central thickness at 540 µm OD and 529 µm OS. Dilated exam was remarkable for a long-standing superior tear with surrounding retinopexy scars.
Specular microscopy showed central confluent guttae in both eyes. Endothelial cell density could not be reliably calculated due to the density of the guttae.
The patient was told that her vision was being limited by both cataract and corneal changes related to Fuchs corneal dystrophy. After discussing treatment options, the patient elected to undergo cataract extraction with placement of a toric IOL and DSO. The IOL power was selected to target -0.50 D to account for the 0.50 D hyperopic shift typically induced by DSO.5
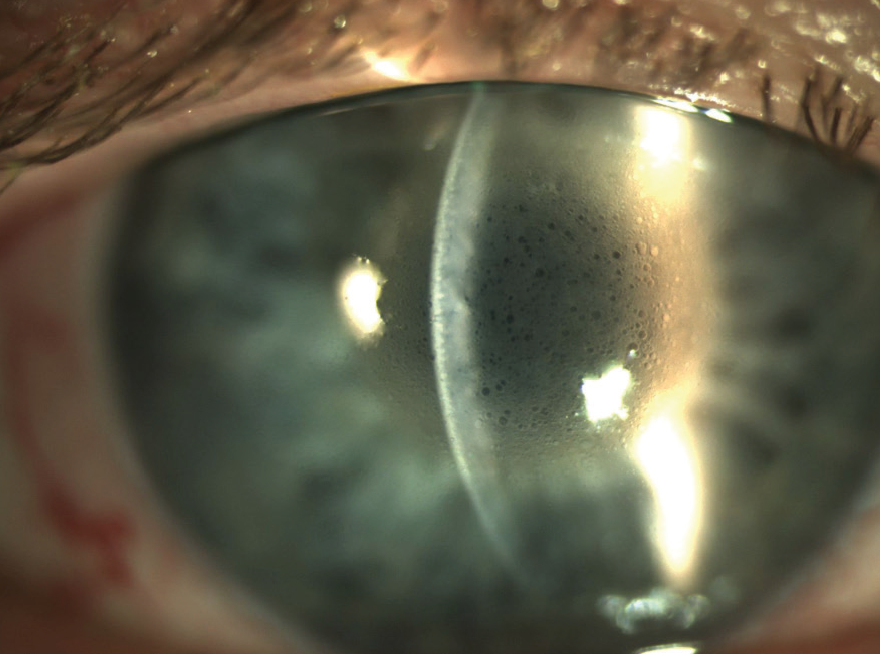
Figure 1. Postoperative corneal findings 1 day after DSO.
On the first postoperative day, UCVA was counting fingers at 5 ft OD. Slit-lamp examination showed a central descemetorhexis with overlying stromal and microcystic edema (Figure 1). The anterior chamber had 1+ cell, and the posterior chamber toric IOL was in place and centered. The patient’s postoperative drop regimen included prednisolone acetate 1%, ripasudil (Glanatec, Kowa), and sodium chloride 5%, each four times daily, and moxifloxacin three times daily (see Educated Decisions).
After 1 week, the patient’s distance UCVA was 20/80 OD, improving to 20/25 with pinhole testing. Slit-lamp exam showed improvement of the microcystic and stromal edema (Figure 2). The postoperative drop regimen was continued without change, although the patient was instructed to stop the moxifloxacin 2 weeks after surgery.
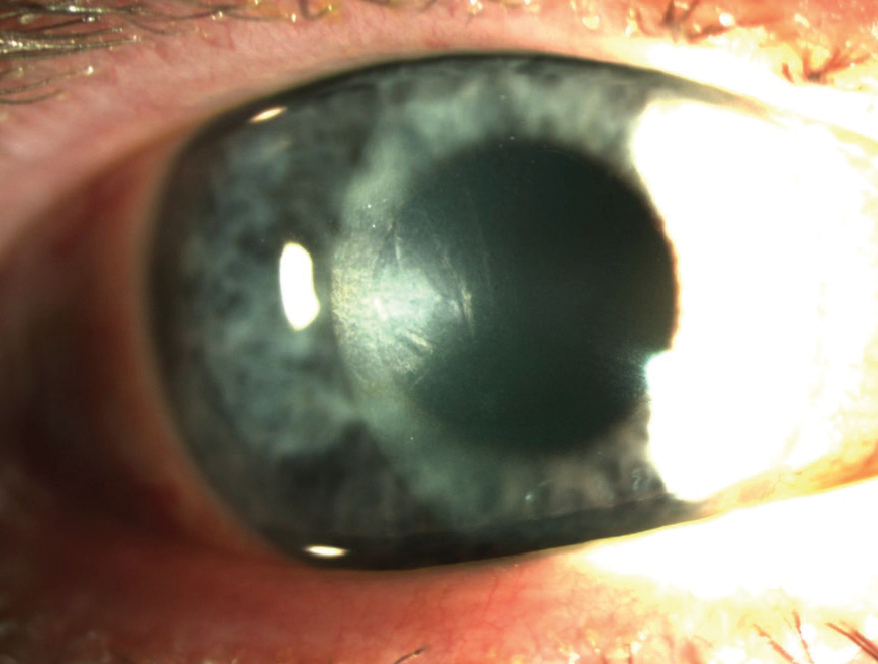
Figure 2. Same eye at 1 week after DSO.
At the 3-month postoperative visit, UCVA had improved to 20/25 OD and BCVA was 20/20 (-0.50 +0.75 x 013°). Slit-lamp exam showed a central descemetorhexis with full resolution of corneal edema (Figure 3). At this time, the patient elected to proceed with the same surgical plan in the left eye.
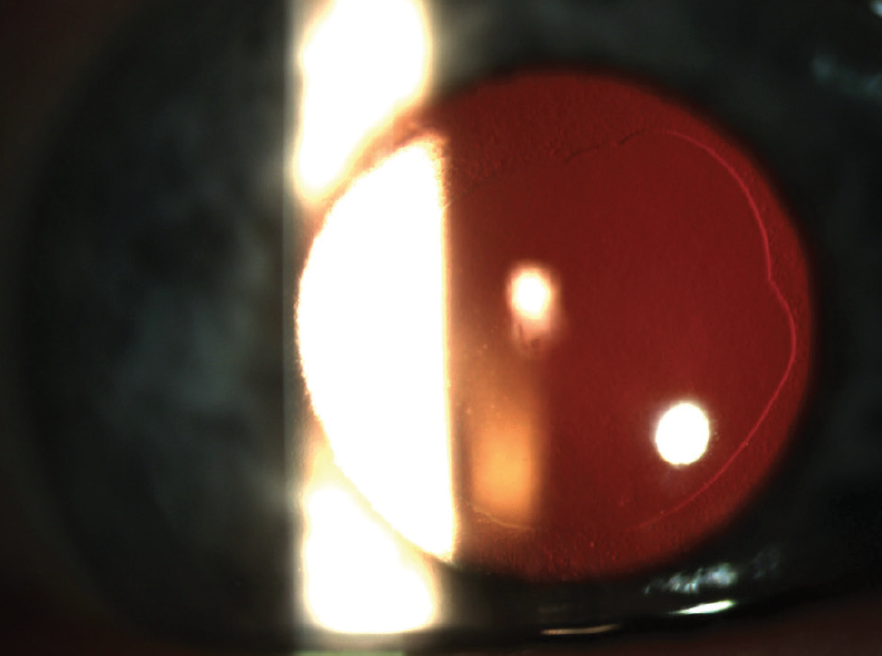
Figure 3. Same eye at 3 months after DSO.
The left eye had a similar immediate postoperative course. On postoperative day 1, acuity was 4/200 with severe corneal edema overlying the central descemetorhexis. At the patient’s most recent follow-up 1 month later, UCVA had improved to 20/30 OS with BCVA of 20/20 (-0.75 +0.75 x155°). The patient’s UCVA in the right eye, now 5 months after cataract extraction with DSO, was 20/20-2. Based on the study by Macsai et al,3 it is presumed that the ripasudil contributed to her rapid visual recovery following DSO.
DISCUSSION
This case and the published literature support the use of DSO as an alternative to keratoplasty for selected patients with Fuchs corneal dystrophy. Appropriate surgical candidates have visually significant central corneal involvement with a relatively clear corneal periphery.
A retrospective comparative cohort study found visual outcomes with DSO to be equivalent to those with DMEK.6 Although the time required for visual recovery following DSO is potentially longer than that for DMEK, DSO has many advantages, including no postoperative positioning requirements, no rejection risk or need for chronic topical steroid therapy, and no need for donor tissue. DSO has demonstrated encouraging short-term outcomes for appropriate candidates, but long-term results are still being investigated.
1. Eye Bank Association of America. Eye Banking Statistical Report. 2018, https://restoresight.org/wp-content/uploads/2019/03/2018_Statistical_Report-Complete-1.pdf. Accessed September 1, 2020.
2. Deng SX, Lee WB, Hammersmith KM, et al. Descemet membrane endothelial keratoplasty: safety and outcomes: a report by the American Academy of Ophthalmology. Ophthalmology. 2018;125(2):295-310.
3. Macsai MS, Shiloach M. Use of topical rho kinase inhibitors in the treatment of Fuchs dystrophy after Descemet stripping only. Cornea. 2019;38(5):529-534.
4. Okumura N, Kinoshita S, Koizumi N. Application of rho kinase inhibitors for the treatment of corneal endothelial diseases. J Ophthalmol. 2017;2017:2646904.
5. Davies E, Pineda R 2nd. Corneal tomography changes and refractive outcomes after Descemet stripping without endothelial keratoplasty. Cornea. 2019;38(7):817-819.
6. Huang MJ, Kane S, Dhaliwal DK. Descemetorhexis without endothelial keratoplasty versus DMEK for treatment of Fuchs endothelial corneal dystrophy. Cornea. 2018;37(12):1479-1483.



