
The integrity of the ocular surface plays an important role in the success of cataract and refractive surgery. Treatment of dry eye disease (DED) is becoming an integral part of primary eye care, and optometrists are poised perfectly to initiate treatments for DED in a primary eye care setting. By identifying and treating DED before surgery, optometrists can help to prime their patients for successful outcomes.
AT A GLANCE
- By identifying and treating dry eye disease (DED) before refractive surgery, optometrists can help to prime their patients for successful refractive outcomes.
- Aggressive treatment of DED can help to control symptoms quickly in patients seeking refractive surgery.
- As comanagement of refractive surgery increases, it is the responsibility of optometrists to detect DED and initiate treatment.
Patient counseling and education is vital to the success of DED treatment. It is widely accepted that DED is largely unrecognized. Often, patients are motivated toward refractive surgery but are unaware of their own contributing signs and symptoms of DED. Reasons for this lack of awareness may include the absence of a diagnosis or patient education. Also, in my experience, patients often incorrectly assign symptoms of DED to a growing cataract and falsely believe that these will resolve after surgery.
Further complicating the matter, even when signs and symptoms are present, they may not correlate. In a study that assessed the presence of dry eye signs and symptoms in patients scheduled for cataract surgery, only 30% of patients reported symptoms of DED, but up to 62% patients had a tear breakup time (TBUT) of less than 5 seconds and 76% had corneal staining. Furthermore, only 22% of patients entered the study with a diagnosis of DED.1
In another study of pre–cataract surgery patients, 57% presented without previous diagnosis of DED and, of this cohort, 80% presented with one clinical sign consistent with DED.2 The study authors also suggested that there is a disconnect between signs and symptoms, as 83% of patients who scored normal on the symptom questionnaire had at least one clinical finding consistent with DED.
There is a need to identify DED in asymptomatic and symptomatic patients who present for refractive surgery. Given that upward of 30 million Americans have DED and that cataract surgery is the most commonly performed ocular surgery,2 there is a huge opportunity for optometrists to identify, educate, and manage patients in a primary care setting.
TWO ALGORITHMS FOR TREATMENT
The treatment algorithm outlined by the Tear Film and Ocular Surface Society Dry Eye Workshop II (TFOS DEWS II) is widely employed by eye care practitioners as an organizational tool for managing DED.3 The American Society of Cataract and Refractive Surgery (ASCRS) has also published an algorithm to address DED specifically in patients who are candidates for cataract and refractive surgery.4 The ASCRS algorithm encourages treatment of visually significant DED because of its potential effects on refractive outcome. The goal is rapid resolution of signs and symptoms.
As comanagement of refractive surgery becomes increasingly prevalent, optometrists can implement the ASCRS algorithm to assist in achieving optimal surgical outcomes. Identifying and managing DED in a primary care optometric setting will help optometrists to build the medical side of their practices, help gain the trust of patients in the long-term, and enhance comanagement relationships with refractive surgeons. Untreated DED can lead to inaccurate postsurgical outcomes, most commonly residual refractive error, worsening of DED symptoms, and increased risk for postoperative endophthalmitis.4,5
Given the rise of point-of-care testing for DED markers such as elevated tear osmolarity or level of matrix metalloproteinase-9 (MMP-9), the ASCRS algorithm particularly emphasizes the application of these tests in preoperative patients, along with slit-lamp examination and assessment of patient symptoms. The authors also acknowledge that there is not one single test to identify all ocular surface disease, so that multiple diagnostic tools may be needed. Point-of-care testing also has some disadvantages, including absence of reimbursement, accessibility issues, cost, and volatility of measurements.4
The use of validated questionnaires is recommended to quantify patients’ symptoms. Signs of DED include increased tear osmolarity, corneal staining, and reduced TBUT, among others.
HOW DOES THE ALGORITHM WORK?
The ASCRS algorithm proposes identification of DED at preoperative visits using a modified Standardized Patient Evaluation of Eye Dryness (SPEED II) questionnaire to determine presence of symptoms. Based on the severity of symptoms, tear osmolarity and MMP-9 testing are performed. When clinical examination is performed to evaluate signs of DED, corneal function is evaluated with staining.
If visually significant DED is present, surgery may be delayed as treatment is initiated (Figure). Once the DED becomes non–visually significant on subsequent examination, the measurements are repeated and surgery may proceed.4
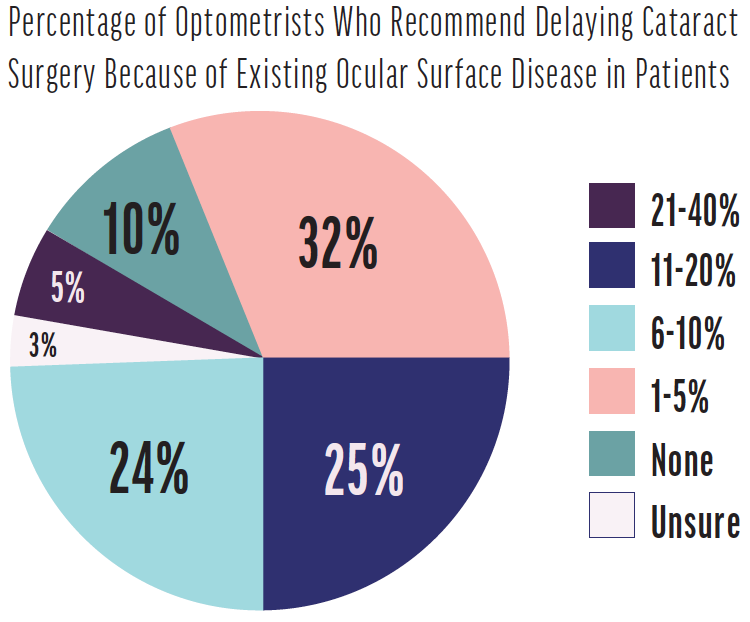
Figure. On average, 8% of comanaged cataract surgery patients are delayed due to ocular surface disease. Source: Modern Optometry 2019 Clinical Survey.
When early cataracts are noted in patients with coexisting DED, there is immense opportunity for optometrists to educate patients regarding their disease process. Education can start with a simple validated questionnaire to review symptoms. Using the TFOS DEWS II criteria for treatment, often implementing Step 1 (environmental changes, changes in medication and diet, and initiation of supplementation with omega-3 fatty acids) will not reverse visually significant ocular surface disease rapidly enough before surgery; therefore, a multifaceted mixed approach of at-home and in-office procedural treatments may be needed as initial treatment. The authors of the ASCRS algorithm propose at minimum initiating treatment at TFOS DEWS II Step 2 for more rapid results, including nonpreserved ocular lubricants, tear conservation with punctal plugs, lubricating ointments, in-office thermal pulsation and intense pulsed light therapy, and prescription topical medications.4
RAPID TREATMENT
Topical corticosteroids are often used to control ocular surface inflammation; if inflammation is uncontrolled, it leads to breakdown of the corneal epithelium.3 The ASCRS treatment algorithm proposes use of corticosteroids to achieve a rapid decrease in tear film cytokines, earlier than would be attempted for a nonsurgical patient.4
Other antiinflammatory therapeutics have also been long used in practice to suppress ocular surface inflammation by decreasing surface T-cell recruitment and activation. Cyclosporine ophthalmic emulsion 0.05% (Restasis, Allergan) has been reported to significantly decrease corneal staining and increase TBUT and visual function out to 6 months.6 Recently, a novel nanomicellar formulation of cyclosporine 0.09% (OTX-101, Sun Pharmaceutical) has shown improvement in corneal staining by as early as 28 days.7
Lifitegrast ophthalmic solution 5% (Xiidra, Novartis) is also used by clinicians to inhibit intercellular activator adhesion molecule-1 (ICAM-1) from binding to the surface of T cells, thereby actively reducing migration and binding of T cells and subsequent release of inflammatory cytokines. In phase 3 studies, lifitegrast was shown to improve ocular dryness within 2 weeks of starting treatment and to improve corneal staining in 12 weeks.8
Treating the Lid Margin
Lid margin disease ideally should be treated as early as possible. At-home treatments such as warm compresses or heat masks, hypochlorous acid products, and re-esterified omega-3 fatty acid supplementation should be emphasized to the patient as maintenance therapy. These types of at-home treatments do not produce rapid results, so they should be implemented as early as possible to prevent advancement of the disease process.
In-office procedures can be used to speed the treatment of lid disease. One such procedure is thermal pulsation (iLux, Alcon), which rapidly restores the homeostasis of the tear film and improves the lipid layer. Thermal pulsation is often used to bypass the limitations of heat transfer to stagnant meibum delivered by warm compresses. In one study, thermal pulsation improved Ocular Surface Disease Index scores and meibomian gland scores (MGS) at 3 months after treatment and sustained these results up to 12 months.9 Improvement in MGS was also associated with shorter duration between diagnosis and treatment and with mild disease, indicating need for earlier treatment to produce superior outcomes.9
For meibomian gland dysfunction, oral azithromycin may produce improvement in vision, conjunctival hyperemia, and corneal staining within 5 days, compared with oral doxycycline, which may take up to 4 weeks.10
Microblepharoexfoliation (BlephEx, BlephEx), performed before thermal pulsation, can effectively reduce bacterial load and possibly reduce the risk of postoperative infection. Intense pulsed light and meibomian gland probing may also be indicated, depending on the disease process.
In addition to providing a more rapid response than at-home treatments, these in-office procedures provide the benefits of improving compliance with treatment and improving ease of treatment for those with physical limitations.
Advanced Treatments
Given the multifactorial nature of DED and the differences in mechanism of action of each type of therapy, optometrists should offer a multitreatment approach to DED in the primary eye care setting.
Advanced treatments, such as use of nonpreserved autologous serum, are reserved for more severe disease. I also find that punctal plugs are often a helpful treatment option for DED.
One perhaps underutilized treatment for keratitis is cryopreserved amniotic membrane (Prokera Slim, Bio-Tissue). The Dry Eye Amniotic Membrane (DREAM) study found that 90% of participants with corneal staining improved significantly with use of cryopreserved amniotic membrane (CAM) within 5 days. The antiinflammatory and healing properties of the CAM facilitate corneal nerve regeneration and corneal reepithelialization, largely attributed to the nerve growth factor present in the membrane.11 The ASCRS Cornea Clinical Committee recommends waiting 7 days between discontinuing amniotic membrane graft placement and proceeding with intraocular surgery.4
Bandage contact lenses have also been used for therapeutic purposes prior to intraocular surgery to heal corneal ulcers and persistent epithelial defects. Judicious use of topical antibiotics should be considered with prolonged use of bandage contact lenses.3
The authors of the ASCRS algorithm also suggest intraocular and/or subconjunctival steroids and antibiotics intraoperatively to reduce the effects of preservative toxicity to the cornea.4 Postoperatively, use of topical medications that allow reduced frequency of instillation can also reduce potential for corneal toxicity.
As comanagement of refractive surgical procedures increases, optometrists should be aware of potential toxicities with topical medications and monitor closely for suboptimal refractive outcomes. If refractive results are suboptimal, or if patients are unhappy with their outcomes, the algorithm should be reinstated, and ocular surface disease should be evaluated and treated aggressively before any further surgical procedures are considered.4
EFFECT ON POSTOPERATIVE OUTCOMES
Worsening of DED after ocular surgery has been reported in the literature.12,13 A study looking at tear meniscus height (TMH), TBUT, Schirmer testing, and symptoms of DED postoperatively found that patients with preexisting DED experienced worse symptoms and decreased TMH up to 2 months postoperatively. Patients who had no preexisting DED developed symptoms and worsening of TMH, TBUT, and Schirmer test postoperatively.12
Intraoperatively, microscopic light exposure and incision placement can also worsen signs and symptoms in the acute postoperative period due to a disrupted corneal nerve plexus and consequent reduced corneal sensation and wound healing.12,14
In my experience, patients who were unaware of or untreated for DED before surgery may incorrectly believe their postoperative symptoms are due to their surgery, and this can contribute to dissatisfaction. Implementing questionnaires, taking external photos, and performing meibography before surgery can help patients understand that their DED is preexisting and forestall postoperative complaints. Optometrists should seek to create a partnership with patients to gain their participation in their treatment regimens.
Residual refractive error is the most common reason for patient dissatisfaction after cataract surgery. In a 2016 retrospective observational study assessing the most common causes of patient dissatisfaction after refractive surgery, 57% of participants were identified as having residual refractive error and 35% as having DED. Of these, 24% were treated for DED and a combined 45% of patients were satisfied with their final outcome.5
Untreated DED can also affect preoperative biometry measurements and topographies in cataract and refractive surgery patients. High tear osmolarity has been correlated with inaccurate and fluctuating keratometry and topography readings, resulting in errors in IOL calculations of >0.50 D in some patients.15 This inaccuracy increases the risk for residual ametropia and refractive surprise postoperatively.
Furthermore, multiple surgeries, such as enhancement surgery to correct residual error, can also worsen an already neurotrophic cornea.
GLAUCOMA CONSIDERATIONS
It has been reported that up to 59% of patients with glaucoma have concurrent ocular surface dysfunction. Worsening of DED symptoms and increased tear film osmolarity have been associated with increasing number of topical medications for glaucoma, duration of treatment, and use of medications that contain the preservative benzalkonium chloride.16,17 Benzalkonium chloride is well known to compromise the corneal epithelium18 and cause conjunctival hyperemia, tear film instability, and subconjunctival fibrosis.19 Additionally, as symptoms of DED increase with the practice of polypharmacy, compliance with glaucoma medications is likely to decrease.
Prostaglandin analogues, the first-line topical treatment for glaucoma for many practitioners, have been reported to aggravate meibomian gland dysfunction.20 Because glaucoma, DED, and meibomian gland dysfunction are more prevalent in the elderly, exacerbation of DED can be caused by multiple etiologies in this population.
The use of selective laser trabeculoplasty (SLT), minimally invasive glaucoma surgery (MIGS) techniques, and novel sustained-release drug devices may reduce the need for topical medications, thus reducing ocular surface toxicity and inflammation. Optometrists can reduce the risk of exacerbating the ocular surface by choosing preservative-free topical medications for their patients with glaucoma and by referring patients for SLT or MIGS when necessary. There are also opportunities for optometrists to comanage patients undergoing these procedures and concomitantly manage their DED.
IS A SURGICAL DELAY REALISTIC?
In my experience, most patients are motivated to participate in the resolution of DED before cataract surgery, especially when optimal outcomes are important. Counseling patients with the assistance of anterior segment photos that highlight corneal staining, measuring noninvasive TBUT, and assessing meibography are all helpful steps for encouraging patient participation.
Optometrists play a crucial role in managing DED in a primary eye care setting. As comanagement of refractive surgery increases in the optometric community, it is our responsibility to initiate DED treatment to facilitate successful refractive outcomes.
1. Trattler WB, Majmudar PA, Donnenfeld ED, McDonald MB, Stonecipher KG, Goldberg DF. The Prospective Health Assessment of Cataract Patients’ Ocular Surface (PHACO) study: the effect of dry eye. Clin Ophthalmol. 2017;11:1423-1430.
2. Gupta PK, Drinkwater OJ, VanDusen KW, Brissette AR, Starr CE. Prevalence of ocular surface dysfunction in patients presenting for cataract surgery evaluation. J Cataract Refract Surg. 2018;44(9):1090-1096.
3. Craig JP, Nelson JD, Azar DT, et al. TFOS DEWS II Report Executive Summary. Ocul Surf. 2017;15(4):802-812.
4. Starr CE, Gupta PK, Farid M, et al. An algorithm for the preoperative diagnosis and treatment of ocular surface disorders. J Cataract Refract Surg. 2019;45(5):669-684.
5. Gibbons A, Ali TK, Waren DP and Donaldson KE. Causes and Correction of Dissatisfaction After Implantation of Presbyopia-Correcting Intraocular Lenses. Clin Ophthalmol. 2016;10:1965-1970.
6. Stonecipher KG, Torkildsen GL, Ousler GW 3rd, Morris S, Villanueva L, Hollander DA. The IMPACT study: a prospective evaluation of the effects of cyclosporine ophthalmic emulsion 0.05% on ocular surface staining and visual performance in patients with dry eye. Clin Ophthalmol. 2016;10:887-895. Published 2016 May 13.
7. Goldberg DF, Malhotra RP, Schechter BA, Justice A, Weiss SL, Sheppard JD. A Phase 3, Randomized, Double-Masked Study of OTX-101 Ophthalmic Solution 0.09% in the Treatment of Dry Eye Disease. Ophthalmology. 2019;126(9):1230-1237.
8. Godin MR, Gupta PK. Lifitegrast ophthalmic solution in the treatment of signs and symptoms of dry eye disease: design, development, and place in therapy. Clin Ophthalmol. 2017;11:951-957. Published 2017 May 22.
9. Blackie CA, Coleman CA, Holland EJ. The Sustained Effect (12 Months) of a Single-Dose Vectored Thermal Pulsation Procedure for Meibomian Gland Dysfunction and Evaporative Dry Eye. Clin Ophthalmol. 2016;10:1385-1396.
10. Benedetti GD, Vaiano AS. Oral azithromycin and oral doxycycline for the treatment of meibomian gland dysfunction: a 9-month comparative case series. Indian J Ophthalmol. 2019;67(4):464-471.
11. McDonald MB, Sheha H, Tighe S, et al. Treatment outcomes in the DRy Eye Amniotic Membrane (DREAM) study. Clin Ophthalmol. 2018;12:677-681. Published 2018 Apr 9.
12. Cho YK, Kim MS. Dry eye after cataract surgery and associated intraoperative risk factors. Korean J Ophthalmol. 2009;23(2):65-73.
13. Ishrat S, Nema N, Chandravanshi SCL. Incidence and pattern of dry eye after cataract surgery. Saudi J Ophthalmol. 2019;33(1):34-40.
14. Donnenfeld ED, Solomon K, Perry HD, et al. The effect of hinge position on corneal sensation and dry eye after LASIK. Ophthalmology. 2003;110(5):1023-1030.
15. Epitropoulos AT, Matossian C , Berdy CJ, Malhotra RP, Potvin R. Effect of Tear Osmolarity on Repeatability of Keratometry for Cataract Surgery Planning. J Cataract Refract Surg. 2015;41(8):1672-1677.
16. Garcia-Feijoo J, Sampaolesi JR. A multicenter evaluation of ocular surface disease prevalence in patients with glaucoma. Clin Ophthalmol. 2012;6:441-446.
17. Labbé A, Terry O, Brasnu E, Van Went C, Baudouin C. Tear film osmolarity in patients treated for glaucoma or ocular hypertension. Cornea. 2012;31(9):994-999.
18. Skalicky SE, Goldberg I, McCluskey P. Ocular surface disease and quality of life in patients with glaucoma. Am J Ophthalmol. 2012;153(1):1-9.e2.
19. Baudouin C, Labbé A, Liang H, Pauly A, Brignole-Baudouin F. Preservatives in eyedrops: the good, the bad and the ugly. Prog Retin Eye Res. 2010;29(4):312-334.
20. Mocan MC, Uzunosmanoglu E, Kocabeyoglu S, Karakaya J, Irkec M. The Association of Chronic Topical Prostaglandin Analog Use With Meibomian Gland Dysfunction. J Glaucoma. 2016;25(9):770-774.



