As the visual needs of a maturing US population increase, surgical and nonsurgical treatment options are expanding rapidly. With an aging population, a large part of most modern optometric practices will include caring for patients who are undergoing cataract surgery and interventional glaucoma surgery by an ophthalmologist.
Not long ago, the typical protocol of glaucoma surgeons was to pursue conservative, nonsurgical treatments such as drops followed by selective laser trabeculoplasty (SLT) before resorting to more invasive surgical solutions, such as trabeculectomy or glaucoma drainage device implantation, which lower IOP effectively but come with significant side effects.
In the area of surgery, microinvasive glaucoma surgery (MIGS) is becoming a standard of care for the treatment of glaucoma in many instances. No longer must surgeons monitor patients until structural and functional damage to the optic nerve is considered severe enough to warrant trabeculectomy or tube shunt, for it is in this subset of patients—beyond laser but not yet warranting invasive surgery—that the advent of MIGS has been most beneficial.
WHAT IS MIGS?
What exactly is MIGS, and why would we encourage patients to undergo it? MIGS is a group of procedures performed using microscopic surgical instruments and devices, often inserted through the same incisions created for the purpose of cataract surgery. Involving minimal tissue disruption, MIGS procedures are very safe, and this is a hallmark of the category (see Defining MIGS). Unlike traditional glaucoma surgeries that were done from outside the eye (ab-externo), MIGS procedures are classically done from the inside (ab-interno).
MIGS has allowed modern eye care providers to dramatically change their approach to the treatment of glaucoma, transitioning from a more passive, conservative medical approach to a more active, interventional approach. As this modality of glaucoma treatment has become increasingly popular, more and more MIGS devices have made their way into surgical suites. This article, although not an exhaustive overview, provides brief updates on several of the MIGS devices, broken down by surgical pathway, now used by our surgeons at Williamson Eye.
THE CONVENTIONAL PATHWAY: SCHLEMM CANAL
iStent and iStent inject
The first generation iStent (Glaukos) is indicated for use in patients with mild to moderate glaucoma who are undergoing cataract surgery. The surgical routine for phacoemulsification is unchanged when the surgeon decides to add an iStent device. Generally he or she would place the iStent through the trabecular meshwork (TM) and into Schlemm canal at the end of cataract surgery.
Although the iStent was successful, a second generation—the iStent inject Trabecular Micro-Bypass System—was developed, and the device was approved last year by the FDA. The iStent inject is the first available ab interno micro-bypass system designed to restore the natural physiologic
outflow using two stents through the TM, as compared to the single stent of the original product. This results in multidirectional flow through Schlemm canal and optimizes aqueous outflow through the natural physiologic pathway, while maintaining an excellent overall safety profile similar to that of cataract surgery alone.
The iStent inject is a 0.4-mm, one-piece, mushroom-shaped stent composed of surgical grade titanium with lateral fenestrations to allow aqueous flow. Its microscopic size allows surgeons to implant more than one device to further lower a patient’s IOP. The stents are designed to stay in the eye permanently.
A randomized multicenter clinical trial that included 505 eyes with mild to moderate primary open-angle glaucoma (POAG) at 41 investigational sites concluded that the iStent inject achieved a statistically significant reduction in unmedicated diurnal IOP in patients who underwent cataract surgery with the device compared to those undergoing cataract surgery alone. At the study’s endpoint of 24 months, patients who had the iStent inject along with phacoemulsification were found to have a mean 13.9% lower IOP than patients undergoing phaco alone.1 The overall rate of adverse events for the iStent inject group was similar to that for the cataract surgery alone group.

Kahook Dual Blade
The Kahook Dual Blade (KDB; New World Medical) is a single-use goniotomy blade designed to perform complete removal of TM through a minimally invasive approach with a clear corneal incision. This can be done using the same incision created for cataract surgery or as a standalone procedure. The procedure does not damage the scleral wall and is not associated with a conjunctival bleb. In this excisional goniotomy method, two parallel incisions are made in the TM and the inner wall of Schlemm canal in an attempt to achieve complete removal of TM with minimal, if any, surrounding tissue damage.

In the traditional goniotomy procedure described by Barkan in the 1930s,2 the TM was incised and the residual TM was left postoperatively, leading to scar formation and inconsistent results. By contrast, modern goniotomy with the KDB has been found to achieve stable IOP lowering over time secondary to more complete removal of the TM.
Sieck et al compared the 12-month success rate, as defined by IOP reduction of at least 20% from baseline or reduction of at least one glaucoma medication, in a combined phaco-KDB procedure group and a KDB-alone group. These investigators reported a 71.8% success rate with the phaco-KDB combination, compared with 68.8% in the KDB-alone group. This manifested as a mean IOP reduction at the 12-month checkup from 20.4 mm Hg to 14.1 mm Hg in the phaco-KDB group and from 20.4 mm Hg to 14.1 mm Hg in the KDB along group.3 The authors concluded that KDB goniotomy has a favorable safety profile and is an effective procedure for reducing IOP either as a standalone procedure or combined with phacoemulsfication cataract extraction.
Omni Glaucoma Treatment System
The Omni Glaucoma Treatment System (Sight Sciences) targets all three points of resistance in the eye’s conventional drainage system, including the TM, Schlemm canal, and the distal collector channels. It essentially combines two well-established MIGS procedures into one, using one device and one corneal incision. The Omni system performs catheterization and transluminal viscodilation of Schlemm canal while also cutting the TM. Similar to other MIGS procedures, this can be done as a standalone procedure or combined with cataract surgery. It uses an ab-interno approach that spares the conjunctiva and sclera of incisions. Unlike many other MIGS procedures, no devices are implanted in the eye.

Hydrus Microstent
Cleared by the FDA in August 2018, the Hydrus Microstent (Ivantis) is designed to treat patients with mild to moderate primary open-angle glaucoma in conjunction with cataract surgery. The device is placed within the Schlemm canal, allowing fluid to flow through the stent to enhance the eye’s natural outflow channel, therefore reducing eye pressure. The Hydrus Microstent creates a bypass through the TM while dilated and serves as a scaffold to the canal to augment outflow. Thus, the Hydrus Microstent represents a next-generation, canal-based MIGS therapy through a unique trimodal mechanism of action. This is the newest device to come to market and our practice plans to offer the innovation to the people of Louisiana.

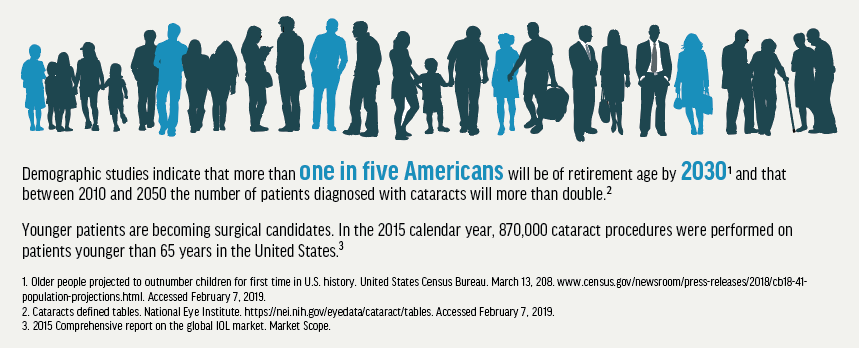
NONCONVENTIONAL OUTFLOW PATHWAYS
Suprachoroidal Space
CyPass Micro-Stent
The CyPass Micro-Stent (Alcon) was approved in 2016 for much the same patient base as the iStent. The CyPass is a fenestrated micro-stent made of biocompatible polyimide material and designed to be inserted into the supraciliary space at the time of cataract surgery. The device creates a permanent conduit between the anterior chamber and the supraciliary space, thereby enabling aqueous drainage through the uveoscleral pathway. Implantation is ab interno using the cataract surgery clear corneal incision.

In August 2018, Alcon announced a voluntary global market withdrawal based on the results of the 5-year postoperative results of the COMPASS-XT safety study. In that study at the 5-year mark, the CyPass Micro-Stent group experienced statistically significant endothelial cell loss compared with a group that had cataract surgery alone. The study also found that the CyPass plus phacoemulsification had a 77% surgical success rate (IOP reduction of at least 20% or reduction of at least one glaucoma drop) at 24 months after surgery, compared with 60% with phacoemulsification alone.4

iStent Supra
Inserted into the eye via the same methodology as the CyPass, the iStent Supra Suprachoroidal Micro-Bypass Stent (Glaukos) is a fenestrated tube composed of titanium and polyethersulfone. It is a 4-mm tube with a hole at each end that is intended to be placed in the supraciliary space. It then allows aqueous fluid to flow through the tube, out through its small holes, and into the uveoscleral space. It can be combined with cataract surgery or done as a standalone procedure.
This suprachoroidal shunt is approved for use in the European Union but is not available in the United States, although the company is pursuing FDA approval. Little has been published about this device, but a presentation at the 2013 European Society of Cataract and Refractive Surgeons meeting indicated that the placement of the iStent Supra resulted in IOP reduction of at least 20% and the ability to discontinue a glaucoma medication in 98% of patients in a study.5

Subconjunctival Pathway
Xen Gel Stent
ffering a robust IOP-lowering efficacy profile not seen in other approved MIGS devices, the Xen Gel Stent (Allergan) has been a game-changer for patients with moderate to severe disease. It can be used alone or in conjunction with cataract surgery, and it is also commonly performed after other MIGS procedures have been attempted. The implant is a gel stent approximately 6 mm in length, with a 45-µm lumen diameter (approximately the size of an eyelash). It is composed of gelatin crosslinked with glutaraldehyde. Upon insertion, the gelatin immediately hydrates and swells to become flexible.
The Xen requires a small corneal incision and is the first ab-interno approach to create a pathway for aqueous flow from the anterior chamber to the subconjunctival space in glaucoma patients. The Xen creates a low-lying, subtle, ab-interno bleb under the conjunctiva. These blebs result in less risk for leaks and infectious complications than standard trabeculectomy blebs. Typically, only one device is implanted in this surgical procedure, and it is designed to stay in the eye permanently.
As a standalone surgery, Xen was found to achieve at least a 20% IOP reduction with fewer or the same number of medications compared to baseline in 76.3% of patients, with a 6.4 mm Hg mean reduction in diurnal IOP from baseline.6
THE MAGIC OF MIGS
Glaucoma treatment with eye drops can be expensive and can have side effects including exacerbation of ocular surface disease. As primary eye care providers, optometrists who are involved in the collaborative care of patients with visually significant cataracts and glaucoma or ocular hypertension can inform these patients regarding safe and efficient MIGS procedures that may reduce their reliance on IOP-lowering drops. In today’s coding vernacular, this could be considered a bundled procedure.
With the advent of MIGS procedures and devices, surgeons can now address two major ocular diseases at once without compromising safety.
- Hengerer FH. Personal experience with second-generation trabecular micro-bypass stents in combination with cataract surgery in patients with glaucoma: 3-year follow-up. Paper presented at: ASCRS Annual Meeting; April 13-17, 2018; Washington.
- Barkan O. Technic of goniotomy. Arch Ophthalmol. 1938;19(2):217-223.
- Sieck EG, Epstein RS, Kennedy JB, et al. Outcomes of Kahook Dual Blade goniotomy with and without phacoemulsification cataract extraction. Ophthalmology Glaucoma. 2018;1:75-81.
- Vold S, Ahmed II, Craven ER, et al; CyPass Study Group. Two-year COMPASS trial results: supraciliary microstenting with phacoemulsification in patients with open-angle glaucoma and cataracts. Ophthalmology. 2016;123(10):2103-2112.
- Junemann J. Twelve month outcomes following ab interno implantation of a suprachoroidal stent and postoperative administration of travoprost to treat open-angle glaucoma. Paper presented at: ESCRS Annual Meeting; October 5-9, 2013; Amsterdam.
- Grover DS, Flynn WJ, Bashford KP, et al. Performance and safety of a new ab interno gelatin stent in refractory glaucoma at 12 months. Am J Ophthalmol. 2017;183:25-36.