Interest in glaucoma management has increased dramatically over the past 10 years, thanks largely to the advent of microinvasive glaucoma surgery. But advances in the field have not been limited to the surgical space. After 2 decades of stagnation, topical glaucoma therapies with new mechanisms of action related to nitric oxide release and rho kinase (ROCK) inhibition came to market a little more than a year ago. Since then, eye care providers have started to figure out where these new medications fit into the glaucoma treatment algorithm and to determine the drugs’ roles in glaucoma surgical management and prevention. This article shares practical pearls and ideas based on our experience with these medications to help other providers judge how to use them.
NITRIC OXIDE DONATION
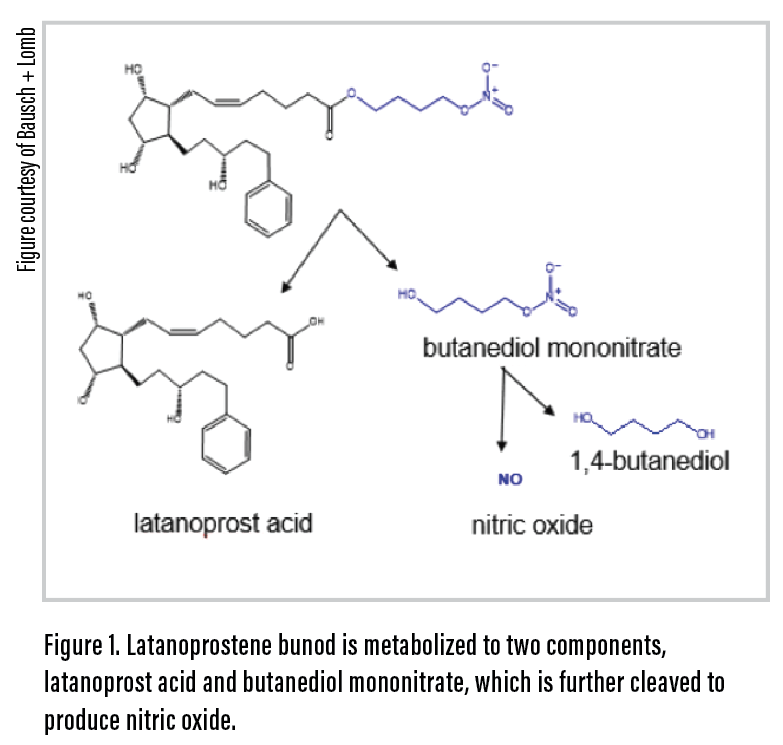
Latanoprostene bunod ophthalmic solution 0.024% (Vyzulta, Bausch + Lomb) is a single molecule that offers a dual mechanism of action, thus enhancing its overall efficacy compared with that of the other prostaglandin analogues (PGAs).1 The product contains latanoprost acid, which, like all PGAs, targets the uveoscleral pathway. In addition, latanoprostene bunod contains a novel subcomponent of nitric oxide, released from broken-down butanediol mononitrate, that directly targets the trabecular meshwork (TM) to relax the TM smooth muscle (Figure 1).
This enhancement of primary trabecular outflow of aqueous humor makes latanoprostene bunod essentially a supercharged PGA, one that lowers IOP by up to 35% from baseline.1 It can restore endogenous nitric oxide that has been shown in studies to be depleted in glaucoma patients. Latanoprostene bunod’s actions can therefore be considered organically restorative with potential long-term advantages that could enhance the longevity of TM outflow.2 Moreover, like other PGAs, the drug is dosed once in the evening, which can enhance patient compliance, and it has a side effect profile similar to that of other PGAs.
Given its strong IOP lowering and simplicity of use, where does latanoprostene bunod fit among the other available medications? We have found this drug to be a highly effective first-line agent like other PGAs. Of note, latanoprostene bunod can reduce IOP whether it is elevated or in the normal range.3 For example, a patient of ours recently diagnosed with primary open-angle glaucoma achieved a decrease in IOP from 34 mm Hg to 17 mm Hg with latanoprostene bunod alone. Another patient of ours presented with glaucomatous progression despite an IOP in the low teens after cataract surgery combined with placement of an iStent Trabecular Micro-Bypass Stent (Glaukos). After 9 months of treatment with latanoprostene bunod, IOP was consistently in the single digits. IOP was decreased by more than 40% in both cases, higher than has been reported in clinical trials. We call patients like these superresponders, and we are actively investigating whether this phenomenon can be classified to permit better targeting.
A more common method of use is switching a patient from another PGA to latanoprostene bunod. We typically apply this strategy when we want to decrease IOP further to meet a target, reasoning that the nitric oxide mechanism may provide the needed boost. This practice is supported by results of the VOYAGER study, which showed a 1.23 mm Hg reduction in IOP compared to latanoprost (Xalatan, Pfizer).1 In our clinical experience, the decrease can sometimes be much higher (range, 2–6 mm Hg), which makes the strategy well worth considering.
A third way to use latanoprostene bunod is as a replacement agent to reduce a patient’s medication burden. For example, we may switch patients using two topical agents such as latanoprost and timolol to monotherapy with latanoprostene bunod. The simplified dosing regimen may improve compliance, and use of a single agent can reduce the preservative load, leading to improvement of the patient’s ocular surface.
ROCK INHIBITION
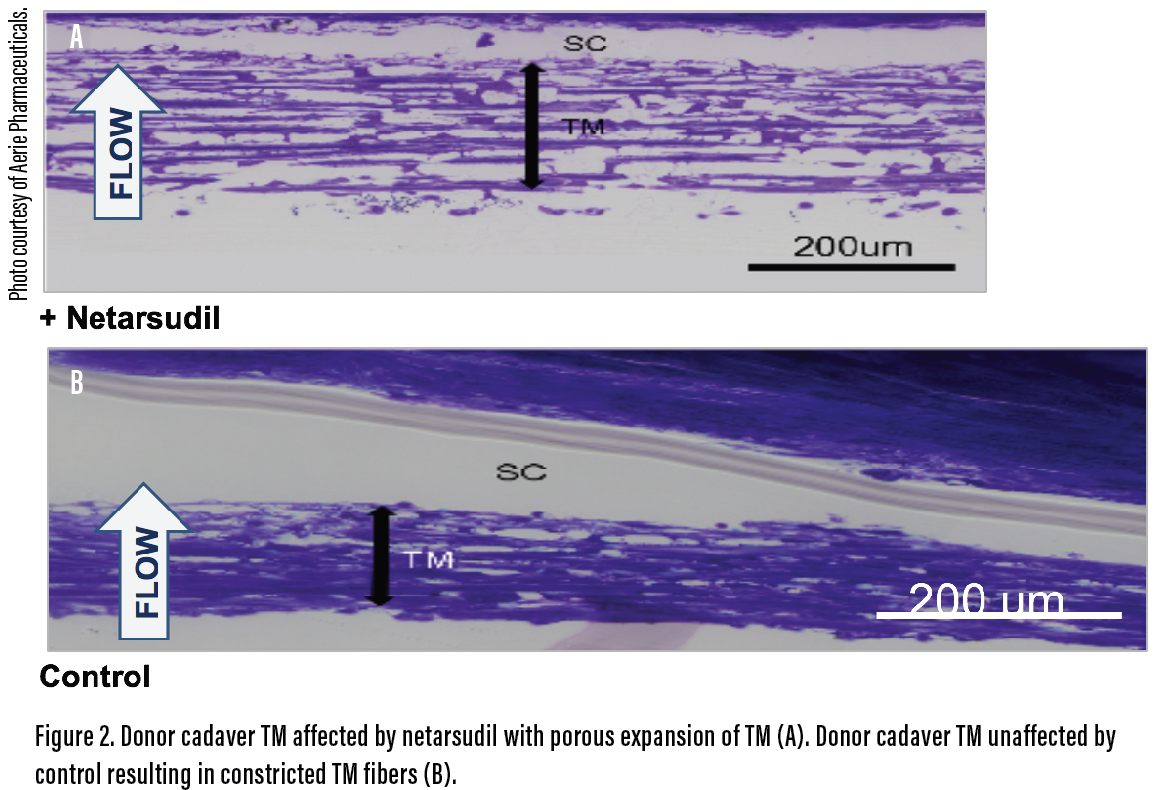
Netarsudil ophthalmic solution 0.02% (Rhopressa, Aerie Pharmaceuticals) is the first of a new class of glaucoma drugs—ROCK inhibitors—to enter the US market. Like latanoprostene bunod, netarsudil acts directly on the TM. It inhibits actin-myosin bonds to relax the TM and improve aqueous outflow (Figure 2). Netarsudil has additional mechanisms that decrease aqueous production and episcleral venous pressure. In the ROCKET studies, the drug decreased IOP by approximately 4 mm Hg to 5 mm Hg and showed efficacy in eyes with ocular hypertension and normal tension, potentially making the drug a first-line option for patients with low baseline IOPs.4
Although we have not typically used netarsudil as a first-line treatment (unless a PGA is not a good option), we have found it to offer consistent efficacy as an adjunctive medication. Like latanoprostene bunod, netarsudil is dosed once in the evening, a simple regimen that in our experience encourages patient adherence to prescribed medical therapy. In situations in which we are hesitant to prescribe a PGA, such as for patients with chronic or active uveitis, netarsudil is an easy and effective alternative. Likewise, we have found netarsudil to be an excellent option for controlling IOP during the early postoperative period after microinvasive glaucoma surgery or filtration procedures when we are concerned about exacerbating inflammation.
As noted earlier, we have prescribed netarsudil for patients with glaucomatous progression for whom other medications are not indicated, for patients who do not tolerate other medications, when IOP is already in the low teens, or when surgery is the next step. Netarsudil also appears to be very synergistic with other glaucoma medications. For example, a 66-year-old man who recently presented to our clinic was using three drops: a fixed combination of dorzolamide and timolol, brimonidine, and latanoprost. His IOP was on target at 12 mm Hg in each eye, but hypertrichosis was interfering with his vision. After substituting netarsudil for latanoprost, his hypertrichosis improved, and in addition his IOP decreased to 9 mm Hg—a welcome development.
Up to 50% of patients treated with netarsudil may develop conjunctival hyperemia,5 so it is important to discuss this possibility before starting therapy. Typically, the hyperemia is mild and decreases over time. Moreover, we have found that, when it occurs, hyperemia can be reduced by combining netarsudil with brimonidine tartrate ophthalmic solution 0.025% (Lumify, Bausch + Lomb). (Note, we have also found that this works for hyperemia from a PGA.) Netarsudil can also cause corneal verticillata to form, which should be noted if seen significantly on clinical examination. Most of us are familiar with seeing corneal verticillata from drugs such as amiodarone. Though not shown to be visually harmful, these corneal effects from netarsudil can be reversed with removal of the medication.
ADDITIONAL POTENTIAL BENEFITS?
Latanoprostene bunod and netarsudil have been welcome additions to our glaucoma armamentarium, and we look forward to using other new agents such as netarsudil 0.02%/latanoprost 0.005% ophthalmic solution (Rocklatan, Aerie Pharmaceuticals), which received FDA approval in March. In the Mercury 1 and 2 pivotal trials, this fixed combination reduced IOP by 1 mm Hg to 3 mm Hg more than monotherapy with either component.6,7
The availability of medications that act directly on the TM and improve its structure and function make us wonder if therapy with these drugs could improve the efficacy of selective laser trabeculoplasty or procedures such as canaloplasty that also work to increase aqueous outflow through the conventional pathway. And will a long-lasting improvement in outflow through the TM slow glaucomatous progression? We hope that future research explores the potential benefits of these new glaucoma medications beyond IOP lowering.
- Weinreb RN, Ong T, Scassellati SB, et al. A randomised, controlled comparison of latanoprostene bunod and latanoprost 0.005% in the treatment of ocular hypertension and open angle glaucoma: the VOYAGER study. Br J Ophthalmol. 2015;99(6):738-745.
- Doganay S, Evereklioglu C, Turkoz Y, et al. Decreased nitric oxide production in primary open-angle glaucoma. Eur J Ophthalmol. 2002;12:44-48.
- Kawase K, Vittitow JL, Weinreb RN, Araie M. Long-term safety and efficacy of latanoprostene bunod 0.024% in Japanese subjects with open-angle glaucoma or ocular hypertension: the JUPITER study. Adv Ther. 2016;33(9):1612-1627.
- Dermatologic and Ophthalmic Drugs Advisory Committee Briefing Document. Aerie Pharmaceuticals. October 13, 2017. www.fda.gov/downloads/advisorycommittees/committeesmeetingmaterials/drugs/dermatologicandophthalmicdrugsadvisorycommittee/ucm579731.pdf. Accessed February 26, 2019.
- Serle JB, Katz LJ, McLaurin E, et al; ROCKET-1 and ROCKET-2 Study Groups. Two phase 3 clinical trials comparing the safety and efficacy of netarsudil to timolol in patients with elevated intraocular pressure: Rho Kinase Elevated IOP Treatment Trial 1 and 2 (ROCKET-1 and ROCKET-2). Am J Ophthalmol. 2018;186:116-127.
- Roclatan Mercury 1 phase 3 topline results [slide set]. Aerie Pharmaceuticals. http://investors.aeriepharma.com/static-files/29defd23-b47b-4319-ba01-5a7ac53e2108. Accessed February 26, 2019.
- Aerie Pharmaceuticals reports positive Roclatan (netarsudil/latanoprost ophthalmic solution) 0.02%/0.005% phase 3 topline efficacy results [press release]. Aerie Pharmaceuticals. July 19, 2017. http://investors.aeriepharma.com/news-releases/news-release-details/aerie-pharmaceuticals-reports-positive-roclatantm-1. Accessed February 26, 2019.