The development of delicate and precise surgical techniques has greatly reduced the risk of complications during cataract surgery over time. Unfortunately, in rare cases adverse events can still occur. In this article we discuss one such complication, retained lens fragments, and offer tips for swiftly identifying and competently managing this potentially serious sequela of cataract surgery.
The goal of cataract surgery is a great visual outcome. The key to achieving this goal centers on removing the crystalline lens while leaving the capsular structure intact, allowing secure placement of an IOL. In an estimated 0.3% to 1.1% of surgeries, however, a piece of the lens remains in the eye.1 This may initiate an inflammatory cascade that can result in various ocular consequences and the need for additional surgery.
WHAT’S THE PROBLEM?
Why does the lens, which has lived in the eye for so long, cause problems if part of it is left behind during surgery? This has to do with the anatomy and genesis of the lens itself.
The three main structures of the lens, the nucleus, the cortex, and the capsular bag, develop by the tenth week of gestation from the ectodermic embryonic layer. The capsular bag sequesters the nucleus and cortex from the body’s immune system throughout our life. Once unencapsulated, the epithelial cells of the crystalline lens become highly antigenic, resulting in inflammation.
Retained cortex and nucleus behave differently after cataract surgery. Cortical material causes low-grade inflammation that can sometimes be managed medically, but nuclear material causes a strong, damaging inflammatory response that almost always requires surgical removal.2
WHO IS AT RISK?
Certain conditions can put patients at higher risk for a retained lens fragment. For instance, some patients can respond differently to anesthesia, becoming confused and less cooperative rather than relaxed. If a patient is moving during surgery despite sedation, there is a higher likelihood that the surgeon will be unable to remove all the lens material as the cornea becomes more cloudy or the iris begins to constrict during long cases.
Structural problems can also add risk. When a patient is missing lens zonules, or if the capsular bag breaks during surgery, a lens fragment can migrate into the posterior chamber. This is more common in patients with a morgagnian cataract, pseudoexfoliation, floppy iris syndrome, or connective tissue disorders such as Marfan syndrome or Ehlers-Danlos syndrome.
Patients with media opacity or other causes for a limited view of the posterior chamber at the slit-lamp examination may have compromised capsular structures without the surgeon’s knowledge. This further complicates the surgery and increases the risk of retained lens fragment. Additionally, limited view through the cornea due to corneal edema or corneal scarring will make a retained fragment in the anterior chamber more likely, as it will be harder for the surgeon to see the anterior chamber angle (Figure 1).
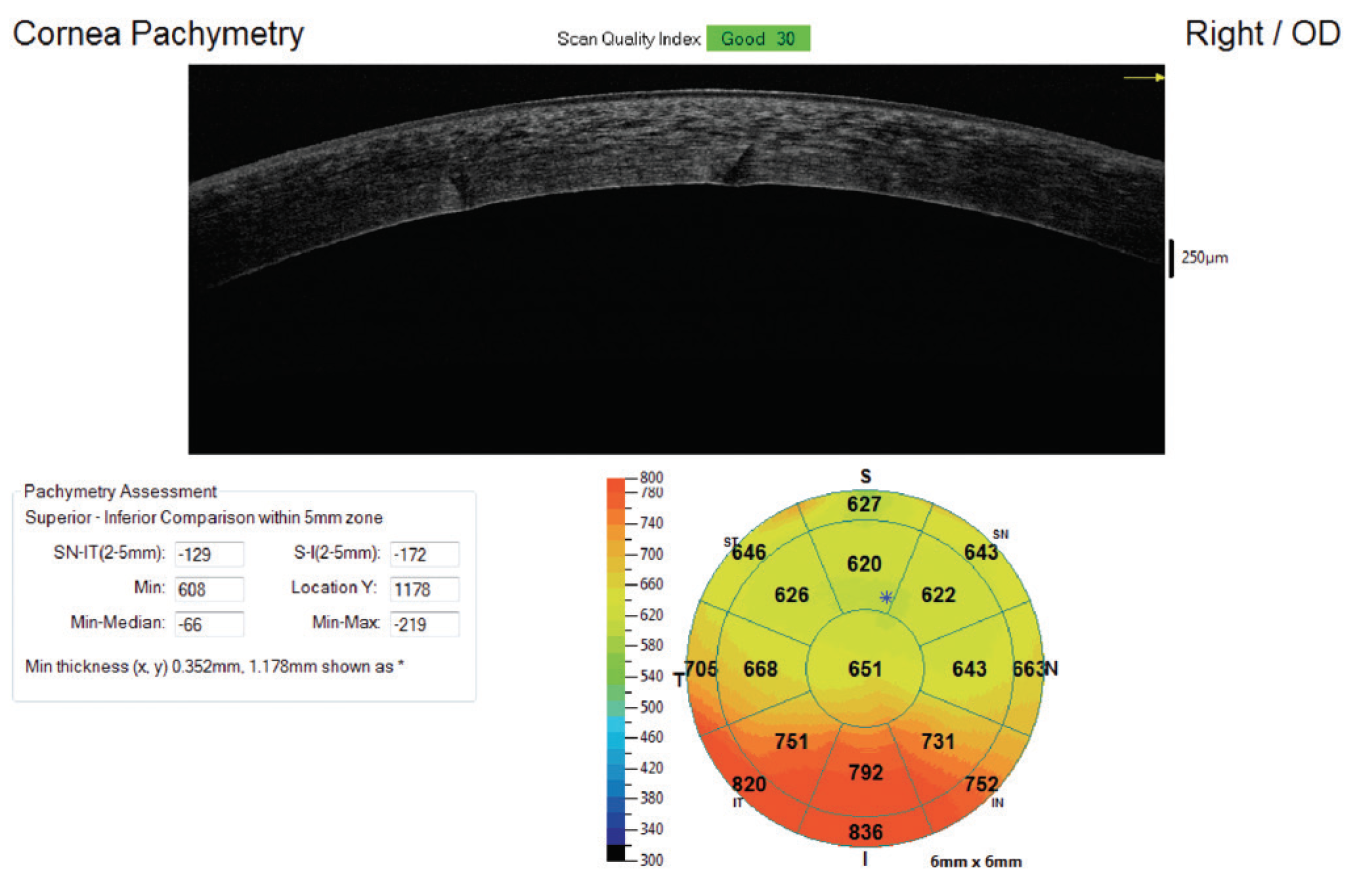
Figure 1. Corneal edema resulting from anterior retained lens fragment. Notice pachymetry mapping shows worse edema inferiorly.
HOW DO WE IDENTIFY A FRAGMENT?
As noted, the body reacts differently to retained cortical and nuclear lens fragments, and the treatment protocols can also be different.
When we observe a nuclear fragment in the anterior chamber or ciliary sulcus, it will have a yellow color and its edges will appear sharp and irregular from the action of phacoemulsification (Figure 2). Retained nucleus will likely result in a rapid autoimmune response with corneal edema and anterior uveitis or panuveitis.
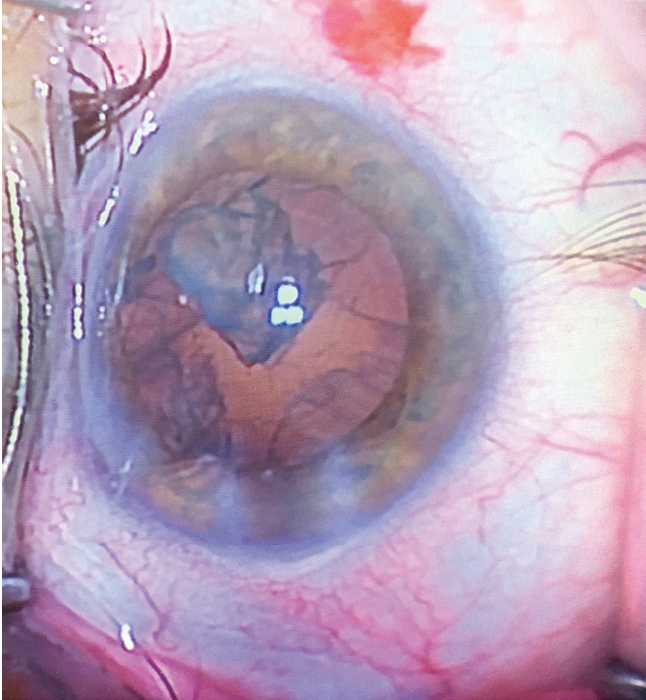
Figure 2. Intraoperative nuclear fragment during phacoemulsification. Note the yellowed and opaque appearance, still with jagged edges.
A retained cortical fragment in the anterior chamber or sulcus will be white, with fluffier, nondiscrete edges. This appearance is due to the disturbance of the collagen structure, the feathery appearance causing immediate whitening and hydration by the aqueous humor (Figure 3). Retained cortex will typically dissolve in 1 to 2 weeks.
If any fragment persists after 2 weeks, the comanaging doctor should suspect that it is nucleus or epinucleus and should send the patient back to the surgeon to have the material removed to prevent late toxicity.
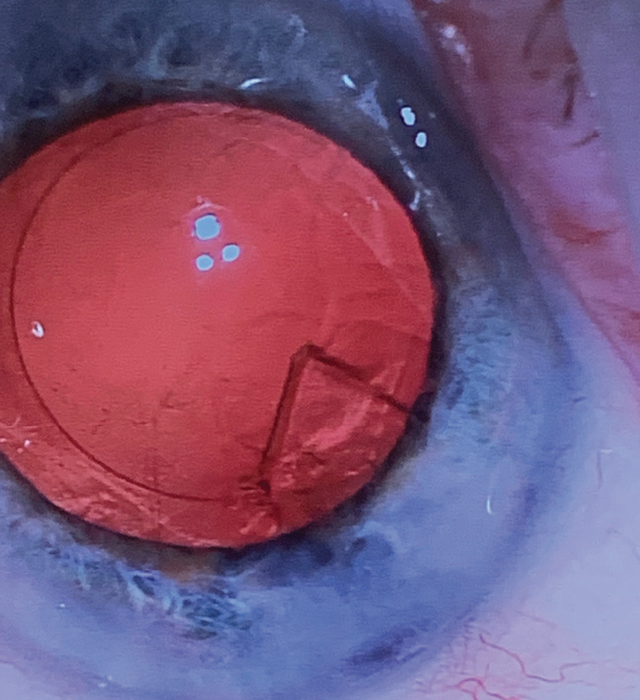
Figure 3. Intraoperative cortical fragment during phacoemulsification. Note clarity of the material. Postoperatively, this material would hydrate and become cloudy with more feathery edges.
In the clinic, a retained lens fragment may be easily noticeable or may be obscured by its location or opaque media. If the cornea is severely edematous, initial visualization of the fragment may be difficult, but important clues will include signs of an acute inflammatory response. These may include worse than typical cell and flare in the anterior and possibly posterior chamber, as well as corneal edema, worse inferiorly.
To achieve a better view in the anterior chamber, the clinician can instill topical filtered 99% glycerin on the surface of the cornea to quickly but temporarily reduce edema. Gonioscopy is also helpful to view the angle before dilation and to look for remaining lens material in the sulcus after dilation. In the case of a broken capsule, which would allow retention in the posterior chamber, a dilated fundus examination may be enough to identify the lens remnant, or B-scan ultrasound can be performed to confirm.
HOW IS THIS PATIENT MANAGED?
Once a retained lens fragment has been identified, it should be removed promptly if there is any suspicion that it is nuclear. Until the time of surgery, patients should be given frequent topical steroids, nonsteroidal antiinflammatory drugs (NSAIDs), and IOP-lowering agents as needed.
The surgeon can remove an anterior lens fragment by performing an anterior chamber washout with either the phacoemulsification handpiece, irrigation/aspiration handpiece, or a soft cannula. Timing of the surgery may be a few days after the fragment is identified, although in the case of a cortical fragment the surgeon may choose to wait up to 2 weeks to allow absorption.
In the event of a retained fragment in the posterior segment, the patient may need to undergo pars plana vitrectomy. The timing of posterior fragment removal is variable. Ideally it would be within 3 days of the initial surgery, but the standard of care is within a few weeks so long as the IOP is within reasonable limits.3 In some instances, the retina surgeon may allow some time for corneal edema to improve before proceeding, in order to get a better view of the posterior chamber.
The most important factor in timing the removal in either anterior or posterior lens retention is IOP. Ideally, the IOP should be maintained below 30 mm Hg to reduce risk of optic nerve damage. If there is poor response to IOP-lowering agents, surgery becomes more urgent.
WHAT ARE THE COMPLICATIONS?
Early complications of a lens fragment in the anterior chamber can include damaged endothelial cells, persistent cell and flare, and corneal edema. Corneal edema is often focal and inferior, but it can be severe enough to obscure view into the anterior chamber. Early complications of a retained lens fragment in the posterior chamber include vitreous cells, cystoid macular edema (CME), corneal edema, and glaucoma.
The longer a fragment causes inflammation, the more extensive the damage. If the lens is in the anterior chamber, damage to the corneal endothelial cells can be too extensive to be compensated for by endothelial cell migration, ultimately requiring endothelial transplant. In this event, the cornea surgeon may first allow the endothelium to recover with topical steroids or sub-Tenon injection of triamcinolone acetonide (Kenalog, Bristol Myers-Squibb) along with hyperosmotic drops (NaCl 5%) for 1 to 2 months before proceeding with surgery.
Posterior retained lens fragments in the long term can lead to CME, prolonged vitritis, and, rarely, retinal detachment. Persistent CME may require treatment for several months, even after the lens fragment is removed (Figure 4). CME can be treated with topical steroids and NSAIDs, and possibly with steroid or anti-VEGF injections.
Phacolytic glaucoma, uveitic glaucoma, or angle-closure glaucoma can also develop as a complication, and these are the most dangerous to ocular health. In most instances, increases in IOP will resolve with removal of the fragment and resolution of the inflammation. In the event of angle closure, however, elevated IOP may persist and require surgical intervention by a glaucoma specialist.
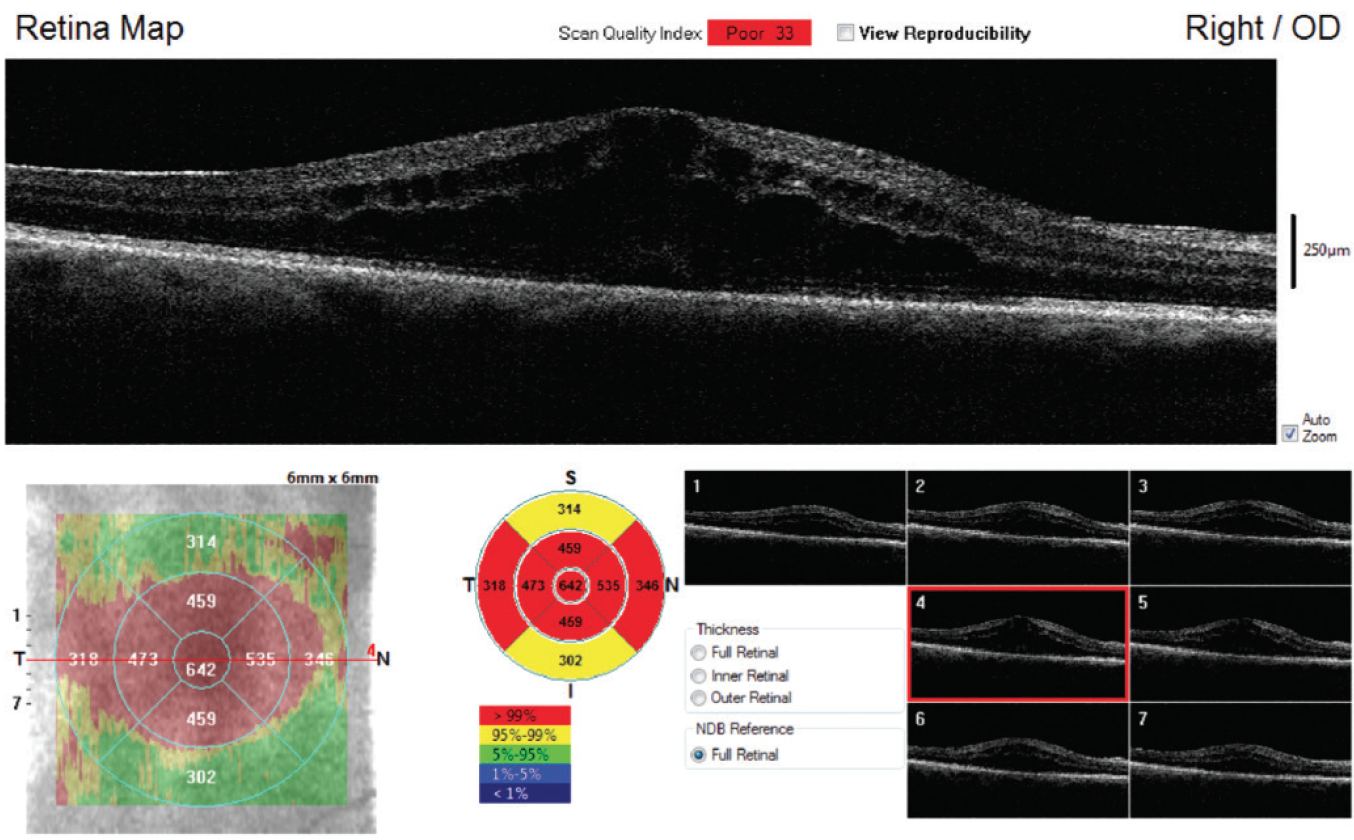
Figure 4. Recurrent CME in a patient who had pars plana vitrectomy after posterior capsular tear that resulted in a retained posterior chamber nuclear lens fragment.
WHAT IS THE OD’S ROLE IN COMANAGING?
The 1-week postoperative visit is an ideal time to look for signs of retained lens fragments, including persistent cell and flare, elevated IOP, corneal edema, and reduced VA. In an uncomplicated case, by 1 week there should be minimal corneal edema, and cell and flare should be resolving. If there is continued inflammation, look for corneal edema, worse inferiorly.
Use gonioscopy to ensure that there is no lens fragment in the angle, and repeat gonioscopy after the patient is dilated to view the sulcus. If you discover a retained fragment, increase the patient’s frequency of topical steroids and NSAIDs to at least four times daily—up to hourly while awake—and refer the patient back to the surgeon.
If the capsule was broken during surgery and a lens fragment fell into the posterior segment, the surgeon may have alerted you to this and to the need for surgical removal. You may need to monitor the patient for CME for months after removal of the fragment.
If you discover a retained lens fragment and you note elevated IOP, add topical antiglaucomatous agents to the patient’s regimen until the IOP can be managed by the surgeon. If glaucoma persists despite fragment removal, the IOP may need aggressive long-term management. If a patient becomes recalcitrant to topical agents, refer him or her to a glaucoma specialist for surgical intervention if necessary.
HOW CAN WE PREVENT THIS PHENOMENON?
Fortunately, retained lens fragments are rare, but certain technologies may help to lessen the risk to your patients. Laser cataract surgery allows more precise fragmentation of the lens and can facilitate its removal, especially in patients at high risk of complications.
As referring physicians, it is important for us to refer patients with higher risk of complications early. For instance, for any patient with weak zonules or a floppy iris, we should consider cataract surgery when there is grade 2 nuclear sclerosis, as opposed to waiting until the lens is in a mature, brunescent stage.
A careful history can identify patients taking an alpha antagonist such as tamsulosin HCl (Flomax, Sanofi-Aventis), and this information can help the surgeon prepare for intraoperative floppy iris syndrome. The surgeon can also use iris hooks or a Malyugin Ring (Microsurgical Technology) to obtain a good view and ensure that no fragments are left in the capsule.
Steps to help the patient remain still during surgery include achieving adequate sedation and pain control, taping the head, or using a retrobulbar nerve block. If the surgeon anticipates an anterior fragment during surgery, he or she can use a cannula to irrigate the angle and flush the sulcus. An endocyclophotocoagulation probe can be used as a miniature endoscope to provide a direct view of the angle in complex cases.
IMPORTANT TO DETECT, BUT TREATABLE
Although retained lens fragments can cause long-term ocular damage, with quick identification the complications are reversible and treatable. Optometrists can prepare their patients for surgery by making sure they know that there can be complications, but that they are rare and manageable with early identification.
1. Moisseiev E, Kinori M, Glovinsky Y, Loewenstein A, Moisseiev J, Barak A. Retained lens fragments: nucleus fragments are associated with worse prognosis than cortex or epinucleus fragments. Eur J Ophthalmol. 2011;21(6):741-747.
2. Nissen SH, Andersen P, Andersen HM. Antibodies to lens antigens in cataract and after cataract surgery. Br J Ophthalmol. 1981;65:63-66.
3. Shah RM, Garg SJ. Options to approach retained lens material. Review of Ophthalmology. April 12, 2011.





