I was already performing research on the cause of variations in surgically induced astigmatism when the institution where I work purchased an intraoperative aberrometer (ORA System; Alcon) in April 2016. At that time, I was also learning more about the influence of posterior corneal astigmatism on refractive results. Naturally, I began using intraoperative aberrometry as part of my efforts to fine-tune my surgical outcomes.
I have found that surgically induced astigmatism can vary from case to case and that the relaxing effect of the cataract incision may not be along the plane of that incision. In other words, it is not possible to use the cataract incision to decrease astigmatism with any kind of accuracy or consistency. Intraoperative aberrometry is the only means by which to directly measure the exact impact of the cataract incision and the power of the anterior and posterior surfaces of the cornea. I rely on this instrument for making my final selection of a toric IOL power in the OR and for refining the IOL’s alignment to minimize residual astigmatism. Two very different cases show the breadth of how useful intraoperative aberrometry can be for customizing astigmatism management.
CASE EXAMPLE NO. 1
A 68-year-old woman came to see me for a cataract surgery evaluation. Upon examination, the patient’s UCVA measured 20/80 OD and 20/60 OS, and her BCVA was plano -4.75 × 180º = 20/60 OD and +1.50 -4.50 × 178º = 20/50 OS. She had a 2+ to 3+ nuclear sclerotic cataract in her right eye and a 2+ nuclear sclerotic cataract in her left eye (Figure 1). Biometry measurements revealed that the patient had a greater amount of with-the-rule astigmatism in both eyes, confirmed by corneal topography (Figure 2), than is correctable by a toric IOL alone, 5.43 D @ 88º OD and 5.36 D @ 84º OS. I therefore recommended a combination of a toric lens implant with possible limbal relaxing incisions. The patient agreed and opted for standard rather than laser cataract surgery. To further refine the astigmatic treatment, I discussed with her using intraoperative aberrometry to guide my surgical plan, including IOL selection.
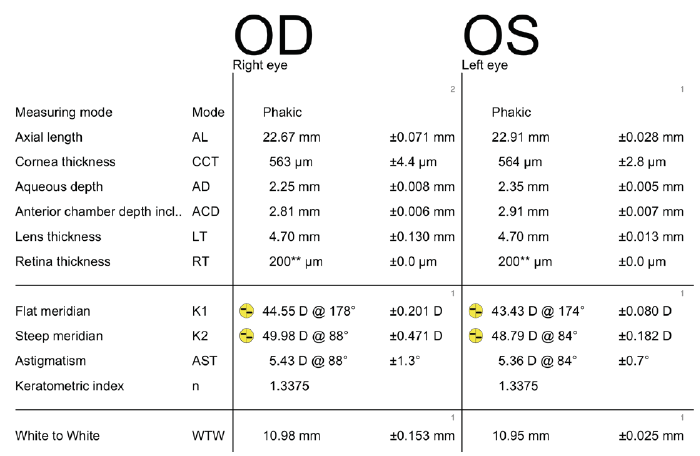
Figure 1. Preoperative biometry with the Lenstar (Haag-Streit).
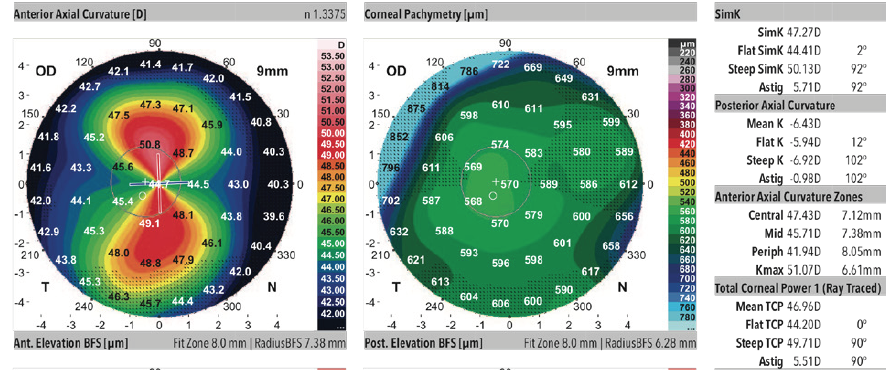
Figure 2. Corneal topography.
I operated on the patient’s right eye first. Intraoperative aberrometry found 4.72 D of astigmatism at 116º and confirmed the preoperative toric calculator recommendation of the 19.50 D model T9 AcrySof IQ Toric IOL (Alcon). After inserting the IOL and optimizing its axial alignment, 1.80 D astigmatism remained. Using intraoperative aberrometry to guide my relaxing incisions, I was able to reduce the final pseudophakic reading to -0.45 D with astigmatism of 0.90 D @ 116º.
Postoperatively, the patient did very well, with 20/20 UCVA at her final 1-month postoperative visit. Her final refraction was +0.25 D sphere and a keratometry reading of 43.77/49.2 @ 89º (5.43).
CASE EXAMPLE NO. 2
A 79-year-old man presented with a complaint of a progressive decline in vision. The patient had a BCVA of 20/30 OU with a refraction of +1.25 -2.00 × 120º OD and a refraction of +1.50 -1.25 × 81º OS that decreased to 20/60 OD and 20/100 OS with glare testing. The astigmatism in his left eye was against the rule, 1.07 D @ 174º (Figure 3). The patient chose to undergo laser cataract surgery and implantation of the AcrySof IQ Restor Multifocal Toric IOL (Alcon).
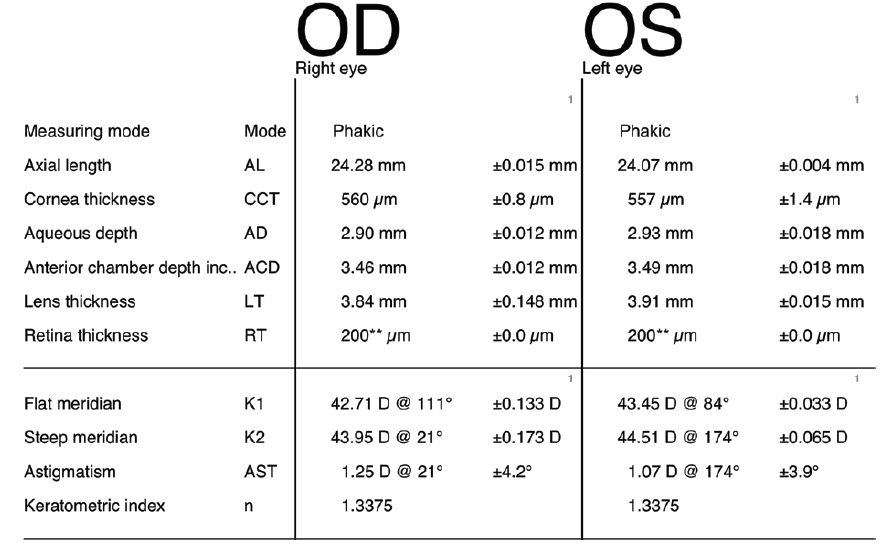
Figure 3. Preoperative biometry with the Lenstar.
Preoperatively, the Barrett Toric Calculator identified the axis of astigmatism at 176º. After the laser portion of the cataract procedure, the intraoperative aphakic aberrometry reading confirmed the preoperative toric power. During the pseudophakic alignment of the toric IOL, however, I changed the preoperative axial recommendation of 176º to the 170º axis, with predicted residual astigmatism of 0.23 D × 143º. Postoperatively, the patient had residual astigmatism of 0.25 D and a UCVA of 20/20 in the operated eye. His final refraction was +0.25 -0.25 × 103º, and the keratometry reading was 44.12/45.18 @ 169º (1.08).



