One of the toughest conversations an eye care provider can have with a patient is explaining that the cost for an FDA-approved treatment for his or her condition is not generally covered by insurance. Unfortunately, this is too often the reality for patients who need to undergo CXL with the »KXL System (Avedro). In CXL, application of a riboflavin 5’-phosphate ophthalmic solution (Photrexa, Avedro) is combined with UV light treatment to strengthen the cornea. This system is approved by the FDA for the treatment of progressive keratoconus, a form of corneal ectasia that results in irregular astigmatism and loss of visual function.
CXL is one way to treat the underlying cause of keratoconus. Alternative treatment options include prescribing rigid or specialty contact lenses, implanting intrastromal corneal ring segments, or performing penetrating keratoplasty (PK). Of these options, only CXL or PK can stop progression of the disease. In some cases, CXL can even slightly reverse the disease’s effects.
Given the costs and the risks associated with a full thickness PK, CXL fills an unmet need. Clinicians at Oslo University Hospital observed that a steep reduction in keratoplasty for keratoconus occurred over a period of years, simultaneous with an increase in CXL treatments (Figure 1). Still, insurance companies have been slow to adopt coverage for CXL despite positive evidence from European and US clinical trials and FDA approval.
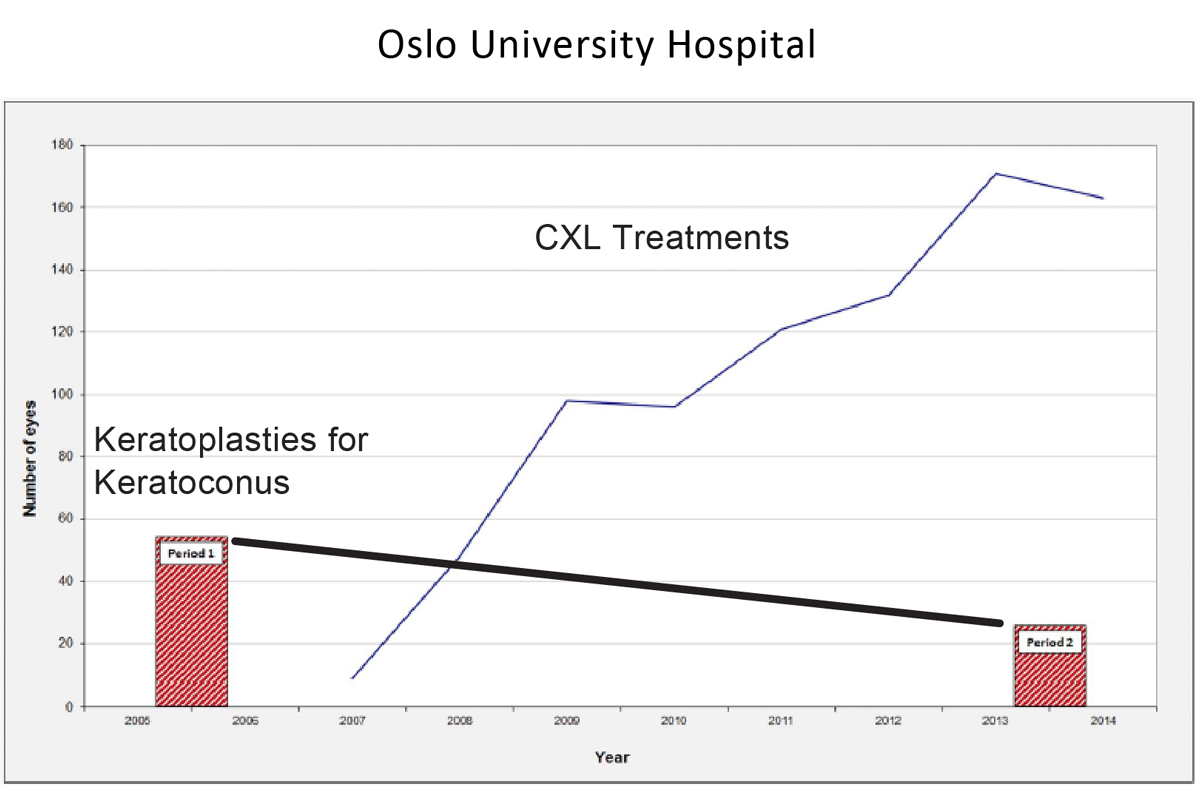
Figure 1. The Oslo University Hospital compared keratoplasty for keratoconus rates for two time periods (2005-2006 and 2103-2014) and found a 53% reduction in the number of procedures. From 2007 to 2014, the same hospital saw the number of CXL procedures rise from fewer than 20 procedures in 2007 to more than 160 in 2014.
It is imperative to identify keratoconus early to halt its progression. This makes keratoconus diagnosis particularly relevant to optometrists. A patient whose BCVA is not crisp should undergo topography. If topography indicates that the patient has keratoconus, then the patient should receive CXL. It is a simple and straightforward algorithm.
THE CONVERSATION
Unfortunately, keratoconus does not affect only people who can afford CXL. Many keratoconus patients are children or young adults without the financial resources to pay for a costly procedure that is not covered by insurance.
I encourage keratoconus patients to lobby their insurance companies to approve coverage; I tell these patients that my staff will do so as well. I explain that such lobbying, in addition to possibly helping their own cases, may help countless others who could also benefit from CXL. In my estimation, this has already started to work: About 18 months ago, almost no CXL procedures performed in my practice were covered by insurance; today, coverage rates have since risen to about 20%.
When a patient’s insurance carrier will not cover CXL despite lobbying efforts, I explain to the patient that a rebate program through Avedro may decrease the price of CXL, and this may make it more financially feasible for the patient to undergo the procedure. When CXL is completely out of reach for a patient regardless of rebate or discount programs, my office will sometimes cover the cost of the procedure in order to protect the patient’s eyes from further disease progression.
DOLLARS AND COMMON SENSE
The cost of riboflavin for one CXL procedure is approximately $2850. Although this might seem high, context is key. Compare the cost of a one-time CXL procedure with the cost of an FDA-approved anti-VEGF injection for retinal disease. The approved drugs cost approximately $2000 per dose and are administered monthly or as-needed following three monthly loading doses.
Further, CXL saves payers from the costs of a PK—and saves patients from the risks associated with such a procedure. PK is risky for the patient and expensive for insurance carriers. When viewed from this perspective, CXL is actually a money saver and risk mitigator. Perhaps insurance carriers will soon realize this, for the benefit of our patients.






