In the United States, small-incision lenticule extraction (SMILE) is approved for the treatment of patients with -1.00 to -8.00 D of myopia, -0.50 D or less of cylinder, and a manifest refraction spherical equivalent of -8.25 D. My fellow researchers and I conducted a straightforward analysis of the preoperative sphere and cylinder in patients who underwent LASIK and met the aforementioned criteria for SMILE. Presented at the SMILE user group meeting held by Carl Zeiss Meditec in New Orleans in November 2017, our findings suggest that SMILE should be applicable to a higher percentage of laser vision correction candidates than originally thought.
OUR FINDINGS
Our retrospective analysis involved a large cohort of previous LASIK cases. The purpose of the study was to assess the potential suitability of SMILE for patients with 1.00 D or more of myopia. The study included 55,461 eyes of 29,677 patients who met the full refractive sphere and age range (22–60 years) specified for SMILE but not the cylinder (manifest astigmatism, 0.00 to -6.00 D) before their LASIK procedure. Patients’ average age was 35 years.
Of all the LASIK patients whose preoperative parameters were within the age and sphere indications for SMILE, 43% had 0.50 D of manifest astigmatism or less in both eyes. Equally important, 19.7% of the total LASIK patients had manifest astigmatism of 0.25 D or less in both eyes. These results indicate that a higher percentage of patients than previously thought may be eligible to undergo SMILE within the currently approved range.
Next, to better understand SMILE’s outcomes in patients with up to 0.50 D of preoperative astigmatism, my colleagues and I reviewed the satisfaction surveys of participants in the US Food and Drug Administration clinical trial that led to the procedure’s approval in 2016. Postoperatively, 329 patients completed a questionnaire as part of the pivotal clinical trial.
Our study analyzed three different aspects of satisfaction: need for glasses or contact lenses to improve postoperative vision, willingness to have the surgery again, and overall satisfaction with the outcome. We reviewed the results for the three preoperative manifest cylinder groups: 0.00, 0.25, and 0.50 D. We found that, 6 months postoperatively, there was no difference in patient satisfaction across these cylinder groups (Figure).
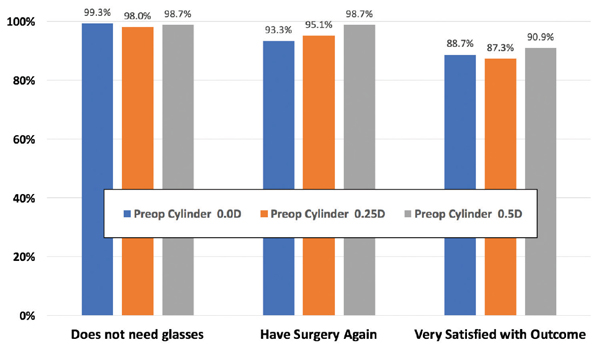
Figure. Patient satisfaction 6 months after SMILE.
CONCLUSION
The results of our analysis should give refractive surgeons a measure of confidence in considering SMILE for patients who have 0.50 D of preoperative astigmatism and meet the other indications for the procedure.
Likewise, the approved SMILE parameters should be applicable in up to 40% of myopic patients who seek refractive surgery and otherwise meet the indications for LASIK.






