Glaucoma is a complex disease that is not fully understood. There have been significant advances in medical and surgical treatment options for glaucoma in recent years. Two new drugs have entered the market in recent months, and a combination agent is in late-stage clinical trials. This article reviews some of the new pharmaceutical options that have shown promise for lowering intraocular pressure (IOP) in patients with glaucoma.
Latanoprostene bunod
Latanoprostene bunod 0.024% (LBN; »Vyzulta, Bausch + Lomb), is a prostaglandin analogue, more specifically a nitric oxide–donating prostanoid FP receptor agonist. It received marketing approval from the US Food and Drug Administration (FDA) in November. The drug has a dual mechanism of action, increasing uveoscleral outflow and relaxing the trabecular meshwork to improve conventional outflow. Dosage is once daily, similar to other prostaglandin analogue medications.
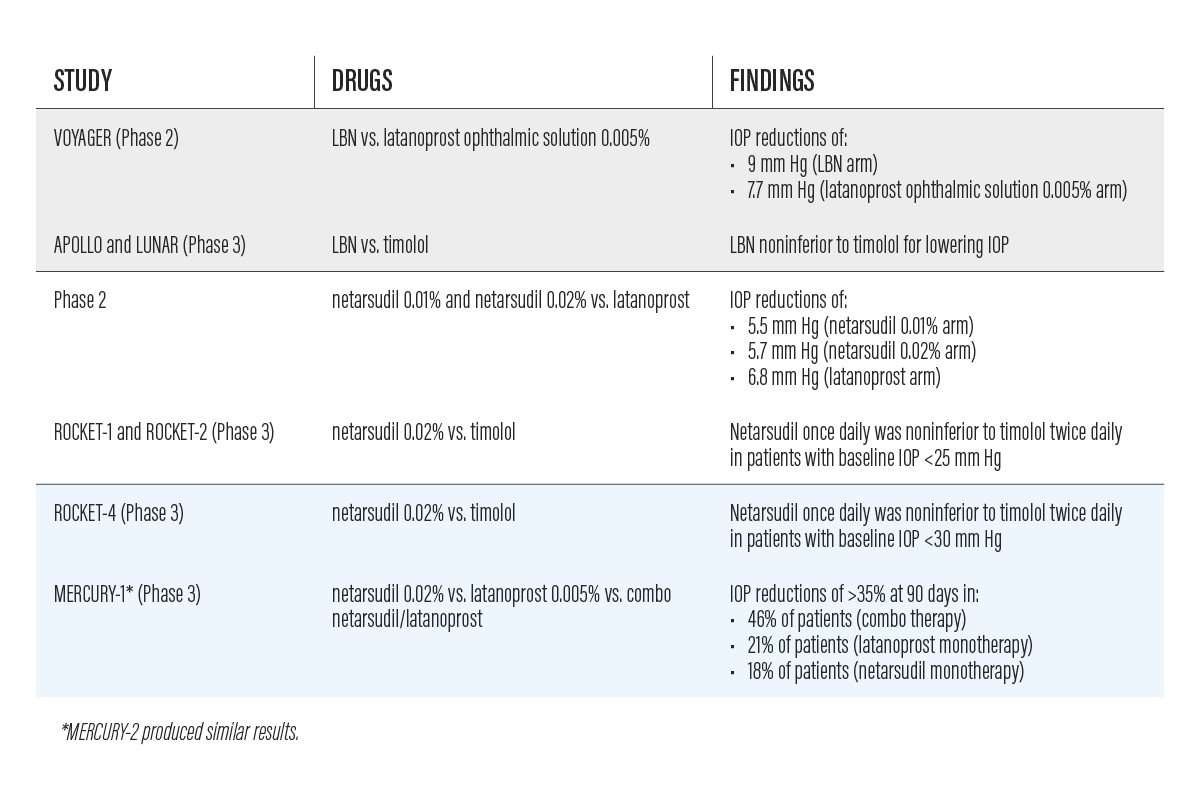
The VOYAGER phase 2 study, comparing LBN to latanoprost ophthalmic solution 0.005%, documented a 9 mm Hg drop in IOP from baseline in the LBN group versus a 7.77 mm Hg drop in the latanoprost 0.005% group.1 The most common adverse event was conjunctival hyperemia, seen in 4.8% of patients in the LBN group.1
APOLLO and LUNAR, a pair of phase 3 noninferiority clinical trials, each demonstrated significantly lower mean IOP with LBN compared with timolol at all time points measured.2,3 Baseline IOP was about 26 mm Hg in both groups. 2,3 The average diurnal IOP at month 3 was 18.1 mm Hg in the LBN group and 19.4 mm Hg in the timolol group, demonstrating noninferiority of LBN to timolol. 2,3
Netarsudil
Netarsudil 0.02% (Rhopressa, Aerie Pharmaceuticals), a rho-kinase inhibitor, received FDA approval in December. Dosed once daily, the drug lowers IOP by blocking rho kinase (ROCK) and the norepinephrine transporter (NET). Inhibition of NET, which occurs in the ciliary body, causes a decrease in aqueous production. Inhibition of ROCK enhances trabecular meshwork outflow. In animal studies, ROCK inhibition showed a vasodilatory effect that reduced episcleral venous pressure.4,5 Clinically, netarsudil could be a useful option as both initial and adjunctive therapy.
A phase 2 study comparing netarsudil 0.01% and 0.02% to latanoprost showed an IOP reduction of 5.5 and 5.7 mm Hg by netarsudil 0.01% and 0.02%, respectively, and of 6.8 mm Hg by latanoprost.
ROCKET-1 and ROCKET-2 were phase 3 clinical trials comparing the safety and efficacy of netarsudil 0.02% and timolol.7 Treatment with netarsudil once daily was noninferior to timolol twice daily in both studies in patients with baseline IOP less than 25 mm Hg.7 The most common adverse event with netarsudil was conjunctival hyperemia, reported in 53% of patients.7 Nine percent of patients reported hyperemia, and investigators reported hyperemia in 46% of patients.
ROCKET-4, another phase 3 clinical trial, revealed similar results to ROCKET-1 and ROCKET-2.8 Netarsudil 0.02% once daily and timolol twice daily caused similar IOP reductions in patients with baseline IOP of less than 30 mm Hg.8
Netarsudil/latanoprost
The fixed combination of netarsudil 0.02% and latanoprost 0.005% (Roclatan, Aerie Pharmaceuticals) is currently being evaluated in clinical trials for FDA approval. Dosed once daily, this combination drug uses multiple mechanisms of action to lower IOP. As noted above, netarsudil decreases aqueous production, enhances trabecular meshwork outflow, and reduces episcleral venous pressure. Latanoprost increases uveoscleral outflow.
Two phase 3 clinical studies, MERCURY-1 and MERCURY-2, compared netarsudil alone versus latanoprost alone, versus netarsudil/latanoprost.9 In Mercury-1, 46% of patients in the combination group experienced IOP reduction of greater than or equal to 35% at 90 days, compared to 21% in the latanoprost monotherapy group and 18% in the netarsudil monotherapy group. The combination drug demonstrated 1 to 3 mm Hg greater IOP lowering than either agent alone, according to an Aerie Pharmaceuticals press release. The most common side effect was conjunctival hyperemia, graded mild and sporadic. MERCURY-2 showed similar results.10
Simple Drops
New formulations of some familiar agents have also recently become available. »Simple Drops (Imprimis Pharmaceuticals) is a suite of compounded preservative-free topical formulations of IOP-lowering drugs. The combination options available make it possible to cut down on the number of bottles that patients need to use. The formulations include Lat (latanoprost), Tim-Lat (timolol/latanoprost), Brim-Dor (brimonidine/dorzolamide), Tim-Brim-Dor (timolol/brimonidine/dorzolamide), Tim-Dor-Lat (timolol/dorzolamide/latanoprost), and Tim-Brim-Dor-Lat (timolol/brimonidine/dorzolamide/latanoprost).
These preservative-free formulations can be an attractive option for patients who experience allergic reactions or hypersensitivity issues with topical glaucoma formulations that contain preservatives.
CONCLUSION
The addition of new pharmaceutical options for our glaucoma patients is a welcomed addition to the glaucoma treatment paradigm. These medications are being shown to be effective at lowering IOP and also well tolerated by patients.
- Weinreb RN, Ong T, Scassellati Sforzolini B, et al; VOYAGER Study Group. A randomized controlled comparison of latanoprostene bunod and latanoprost 0.005% in the treatment of ocular hypertension and open angle glaucoma: the VOYAGER study. Br J Ophthalmol. 2015;99(6):738-745.
- Weinreb RN, Scassellati Sforzolini B, et al. Latanoprostene bunod 0.024% versus timolol maleate 0.5% in subjects with open-angle glaucoma or ocular hypertension: the APOLLO study. Ophthalmology. 2016;123(5):965-973.
- Medeiros FA, Martin KR, Peace J, et al. Comparison of latanoprostene bunod 0.024% and timolol maleate 0.5% in open-angle glaucoma or ocular hypertension: the LUNAR study. Am J Ophthalmol. 2016;168:250-259.
- Alm A, Camras CB, Watson PG. Phase III latanoprost studies in Scandinavia, the United Kingdom and the United States. Surv Ophthalmol. 1997;41(Suppl 2):S105-110.
- Ren R, Li G, Le TD, et al. Netarsudil increases outflow facility in human eyes through multiple mechanisms. Invest Ophthalmol Vis Sci. 2016;57: 6197-6209
- Bacharach J, Dubiner HB, Levy B, et al. Double-masked, randomized, dose-response study of AR-13324 versus latanoprost in patients with elevated intraocular pressure. Ophthalmology. 2015;122(2):302-307.
- Serle JB, Katz LJ, McLaurin E, et al. Two phase 3 clinical trials comparing the safety and efficacy of netarsudil to timolol in patients with elevated intraocular pressure: rho kinase elevated IOP treatment trial 1 and 2 (ROCKET-1 and ROCKET-2). Am J Ophthalmol. 2018;186:116-127.
- Khouri AS, Heah T, Kopczynski CK, et al. A double-masked, randomized, parallel study of netarsudil ophthalmic solution, 0.02% QD compared to timolol maleate ophthalmic solution, 0.5% BID in patients with elevated intraocular pressure (ROCKET-4). Paper presented at: Association for Research in Vision and Ophthalmology Annual Meeting; May 9, 2017; Baltimore.
- Roclatan Mercury 1 Phase 3 Topline Results [investor presentation]. Aerie Pharmaceuticals. Accessed March 13, 2018. http://investors.aeriepharma.com/static-files/f4a8a03c-e34e-4017-93db-5b5d17e2bd50.
- Aerie Pharmaceuticals reports positive roclatan (netarsudil/latanoprost ophthalmic solution) 0.02%/0.005% phase 3 topline efficacy results [press release]. Aerie Pharmaceuticals; Irvine, CA; May 24, 2017.