Dry eye disease (DED) is not completely understood. There are often discrepancies and inconsistencies between a given patient’s clinical signs and his or her reported symptoms.1-3 As diagnostic testing for DED has advanced, so has our understanding of the reasons why the presentations of this common ocular disease can be so variable.4-6
The discrepancy between symptoms and signs can sometimes be so pronounced that clinicians have theorized there is a neurologically based ocular syndrome underlying some patients’ ocular disorders.3,7-9 This has been described with phrases such as symptoms without signs, pain without stain, and even phantom corneal pain.7,6
Recently, the most comprehensive peer-reviewed publication on DED to date, the TFOS DEWS II report, was published.1,9 Part of the TFOS DEWS II report, generated by a comprehensive committee of researchers and clinicians, was a Pain and Sensation Report.9 This committee’s objective was to “highlight the neurobiological mechanisms that underpin discomfort accompanying dry eye disease.” 9 The report acknowledges and defines a category of patients who experience neuropathic pain. 9 The authors noted that this is a separate diagnosis from DED, although the two may be interrelated. Patients with neuropathic pain tend to be some of the most severely symptomatic patients, and unfortunately they show little to no response to treatment employing our traditional arsenal of therapies for DED. 3
Neural pathways and pain perception are not a common topic of discussion at most optometric and ophthalmologic dinner parties. I’d like to start a conversation here today in hopes of laying the groundwork for a better understanding of why some patients perceive their symptoms in the ways that they do.
WHAT IS PAIN?
The International Association for the Study of Pain defines pain as “an unpleasant sensory and emotional experience associated with actual or potential tissue damage, or described in terms of such damage.” 9
Please take note: Pain is not just a physical experience but also an emotional response. Additionally, pain does not have to be associated with actual damage; it can be associated with the potential or possibility of damage. It can also arise from perceived damage, even if none has actually occurred. The latter (symptoms without signs) is defined as neuropathic pain.8,9
Generally speaking, corneal pain arises from the stimulation of nociceptors. Tissue damage activates the nociceptive nerve fibers, and pain signals are released and transmitted up the neural pathways, initiating the pain response including signaling the central nervous system.9 The peripheral nerves of the cornea congregate in two main areas: the stromal plexus, which is in the anterior third of the cornea stroma; and the subbasal nerve plexus, located between the basal epithelium and Bowman layer.3,8-10 The subbasal plexus is by far the densest layer and is easily imaged with confocal microscopy.10-12
Peripheral sensitization refers to a change in the peripheral neurons from detecting and signaling noxious stimuli to signaling nonnoxious stimuli.8,9 When similar changes occur in the central nervous system in response to repeated nerve stimulation, this is known as central sensitization. 8,9 These processes can lead to neuropathic pain.
TREATMENT OPTIONS
Treatment options for patients with neuropathic pain are limited. Autologous serum drops and other blood-derived growth factors seem to be leading the way in addressing peripheral neuropathic eye pain.8-10,13 Amniotic derivatives may also play a role in nerve regeneration, and they continue to evolve as therapeutic options in eye care.14 Scleral contact lenses can also be used to shield eyes with hypersensitive nerves.14
Confocal microscopy has great potential to help document the source of peripheral neuropathic pain (Figure). The ability to obtain details regarding nerve density, tortuosity, neuromas, and beading can help researchers, physicians, and patients work together to improve and heal these delicate neurons.9-12,15 Hopefully, just as other imaging technologies have improved exponentially in the last decade, so will our ability to document and monitor these neurologic pathologies.
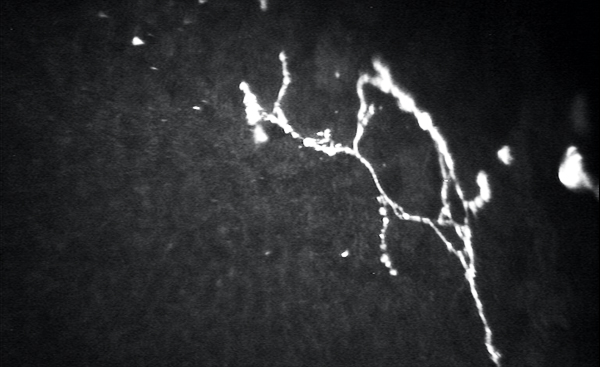
Figure. Confocal microscopy of the basal nerve plexus demonstrating corneal beading, branching, and neuromas in a patient with neuropathic corneal pain.
More centralized neuropathic pain tends to respond to gabapentin or one of its derivatives; these agents can help to decrease allodynia and hyperalgesia.8,14
Neurostimulation has also been shown to be useful in the treatment of pain.14,16,17 The availability of the »TrueTear device (Allergan) has recently brought this therapeutic approach into the realm of ophthalmology.16,17 Other therapies such as acupuncture, meditation, and even intense exercise may have some role in treatment as well.8 One thing is for certain: There is still much we don’t know regarding these complex cases of neuropathic pain.
COLLABORATING IN AND OUTSIDE EYE CARE
Because the treatment of neuropathic eye pain can be so challenging and multifaceted, and the pain itself so persistent, it is important that eye care providers collaborate with a dry eye specialist accustomed to discussing the options and the expected course of therapy with these sometimes frustrated patients.
Eye care providers can collaborate not only within the world of eye care, but also outside our usual referral patterns, reaching out to include pain specialists and pain clinics. This can be particularly important when centralized neuropathic pain is suspected.8 Mental health specialists can help in some cases.9,14 We need to remember that the definition of pain includes a component of emotional experience, and if this is not addressed it may slow improvement or even prevent the patient from improving.
Pain, and especially neuropathic pain, will continue to be a clinical challenge. Hopefully this short introduction has provided a primer to understanding the processes underlying some of these conditions. With expanded understanding, along with advances in diagnostic and therapeutic options, we will be better able to care for these patients.
- Craig JP, Nelson JD, Azar DT, et al. TFOS DEWS II report executive summary. Ocul Surf. 2017;15(4):802-812.
- Bron AJ, Tomlinson A, Foulks GN, et al. Rethinking dry eye disease: a perspective on clinical implications. Ocul Surf. 2014;12(2 Suppl):S1-31.
- Galor A, Levitt RC, Felix ER, et al. Neuropathic ocular pain: an important yet underevaluated feature of dry eye. Eye (Lond). 2015;29(3):301-312.
- Baudouin C, Aragona P, Messmer EM, et al. Role of hyperosmolarity in the pathogenesis and management of dry eye disease: Proceedings of the Ocean Group meeting. Ocul Surf. 2013;11(4).
- Bron AJ, de Paiva CS, Chauhan SK, et al. TFOS DEWS II pathophysiology report. Ocul Surf. 2017;15(3):438-510.
- Craig JP, Nichols KK, Akpek EK, et al. TFOS DEWS II definition and classification report. Ocul Surf. 2017;15(3):276-283.
- Shtein RM, Harper DE, Pallazola V, et al. Discordant dry eye disease (an American Ophthalmological Society thesis). Trans Am Ophthalmol Soc. 2016;114:T4.
- Goyal S, Hamrah P. Understanding neuropathic corneal pain—gaps and current therapeutic approaches. Semin Ophthalmol. 2016;31(1-2):59-70.
- Belmonte C, Nichols JJ, Cox SM, et al. TFOS DEWS II pain and sensation report. Ocul Surf. 2017;15:404-437.
- Aggarwal S, Kheirkhah A, Cavalcanti BM, et al. Autologous serum tears for treatment of photoallodynia in patients with corneal neuropathy: efficacy and evaluation with in vivo confocal microscopy Ocul Surf. 2013;13(3):250-262.
- Cruzat A, Qazi Y, Hamrah P. In vivo confocal microscopy of corneal nerves in health and disease. Ocul Surf. 2017;15(1):15-47.
- De Silva MEH, Zhang AC, Karahalios A, et al. Laser scanning in vivo confocal microscopy (IVCM) for evaluating human corneal sub-basal nerve plexus parameters: protocol for a systematic review. BMJ Open. 2017;7:e018646.
- Pan Q, Angelina A, Zambrano A, et al. Autologous serum eye drops for dry eye. Cochrane Database Syst Rev. 2013;(8):CD009327.
- Jones L, Downie LE, Korb D, et al. TFOS DEWS II management and therapy report. Ocul Surf. 2017;15(3):575-628.
- Patel DV, Mcghee CN. In vivo confocal microscopy of human corneal nerves in health, in ocular and systemic disease, and following corneal surgery: a review. Br J Ophthalmol. 2009;93(7):853-860.
- Gumus K, Schuetzle KL, Pflugfelder SC. Randomized controlled crossover trial comparing the impact of sham or intranasal tear neurostimulation on conjunctival goblet cell degranulation. Am J Ophthalmol. 2017;177:159-168.
- Osenbach R. Neurostimulation for the treatment of intractable facial pain. Pain Med. 2006;7(S1):126-136.






