On a recent Monday in clinic, a 67-year-old patient with severe hypertensive retinopathy pulled out his smartphone and showed me his blood pressure data from the past 4 weeks. He had been using the Nokia BPM+ (Nokia) compact wireless blood pressure monitor at home.
That same day, an 81-year-old patient with exudative age-related macular degeneration (AMD) presented on an emergency basis with a new submacular hemorrhage and catastrophic loss of vision. Because this woman was on concurrent warfarin sodium (Coumadin; Brystol-Myers Squibb) therapy, I worried that perhaps her anticoaugulation parameters were too high and were contributing to the bleeding. But she uses the CoaguChek XS (Roche) meter to regularly monitor her international normalized ratio at home, so I was able to dismiss that as a cause.
Later that week, a 67-year-old patient with diabetes in whom I had suspected obstructive sleep apnea syndrome returned to say that yes, indeed, he had been found to stop breathing 60 times an hour while being monitored via a home-based sleep study.
Then on Friday, I saw an established patient with AMD who was added to my schedule on an emergent basis because of an alert we received from the patient’s ForeseeHome (Notal Vision) AMD Monitoring Program.
This was a typical week in clinic for me. Typical for 2018, but not typical for 10 or even 5 years ago. Technology has slowly but surely crept into the everyday practice of retina specialists and other doctors. Telemedicine was once considered the future, but it is now the present.
THE MVT APP
The mVT app (myVisionTrack; Vital Art and Science) is an application that was cleared by the US FDA and is available for use on Apple and Android smartphones. With a doctor’s prescription, patients can use mVT to monitor themselves at home for any significant vision changes, including those associated with AMD. The patient’s prescribing physician is alerted when changes are detected.
The testing relies on shape discrimination hyperacuity testing. Four circles are displayed on the smartphone screen, but one of the circles is distorted. The patient simply touches the circle with the most distortion. Each time the correct circle is picked, the amount of distortion in the next display is reduced. After correct selection in four or five displays, the distortion may be so slight that the patient selects an incorrect circle. Once the patient misses the correct answer, the distortion is increased until the patient sees it again. After several cycles, the test determines the smallest amount of distortion that can be repeatedly identified. Any change in this result from one session to another suggests that the patient’s AMD may have changed, and an alert is sent to the prescribing physician.
It is recommended that the mVT app be used twice a week for approximately 2 to 3 minutes on each eye. The app costs the user $19.95 per month. There are many free vision tests available on the internet or as downloadable apps, but the mVT app ensures that the prescribing physician is involved and receives any abnormal test results via his or her choice of a portal, email, or phone.
mVT has met the rigorous bar set by the FDA for approval of medical devices. It does not, however, ensure regular use by the patient. On the physician portal, patients using the device are categorized as active, active nontesting, or nonactive. In general, patients approach their retina specialists with information from the company’s website and ask for the physician’s assistance in receiving a starter kit and 10-digit prescription code.
THE PAXOS TELEHEALTH SOLUTION
Paxos (DigiSight Technologies) is a connected HIPAA-compliant eye examination and care coordination tool that allows health care teams to collaborate, in real time, from initial patient encounter to electronic health record documentation, and allows patients to test their visual acuity at home so that physicians can track their progress between visits. Patient information, including images and video, is captured and automatically shared from the point of care. Paxos’ vision assessment offerings are clinically validated tests such as Snellen visual acuity and dynamic Amsler grid testing. Each of these tests has qualified for FDA registration as a class 1 medical device exemption, as they are not significantly different from preceding tests. Paxos Checkup, a home monitoring tool, is currently in clinical trials.
FORESEEHOME
Like the mVT app, the ForeseeHome AMD Monitoring Program has received clearance from the FDA, provides home testing capability using hyperacuity principles, and requires a doctor’s prescription. Unlike the mVT app, ForeseeHome’s benefit in the early detection of choroidal neovascularization due to AMD has been validated in a large prospective randomized clinical trial sponsored by the National Institutes of Health.
The Age-Related Eye Disease Study (AREDS) HOME Study included 1,520 patients with intermediate dry AMD, randomly assigned to use of the ForeseeHome device plus standard care (ie, Amsler grid monitoring and routine clinic visits) or to standard care alone.1 It was 16 times more likely for neovascular AMD to be detected by a ForeseeHome device alert than during a routine office visit.2 In fact, the ForeseeHome device was so advantageous over standard care in the early detection of choroidal neovascularization due to AMD that the HOME Study was terminated early and control patients were offered access to the device. Three papers in the peer-reviewed literature document the findings of the AREDS2 HOME Study.1-3 This validation has led to insurance coverage for the ForeseeHome device in most markets.
The ForeseeHome device takes the final, necessary step in the field of remote patient monitoring. It closes the loop in compliance. Because patient testing is monitored in real time, any lapse in testing is detected, and patients can be called or contacted to alert them to resume testing. Ideally, patient testing should be completed on 5 out of 7 days.
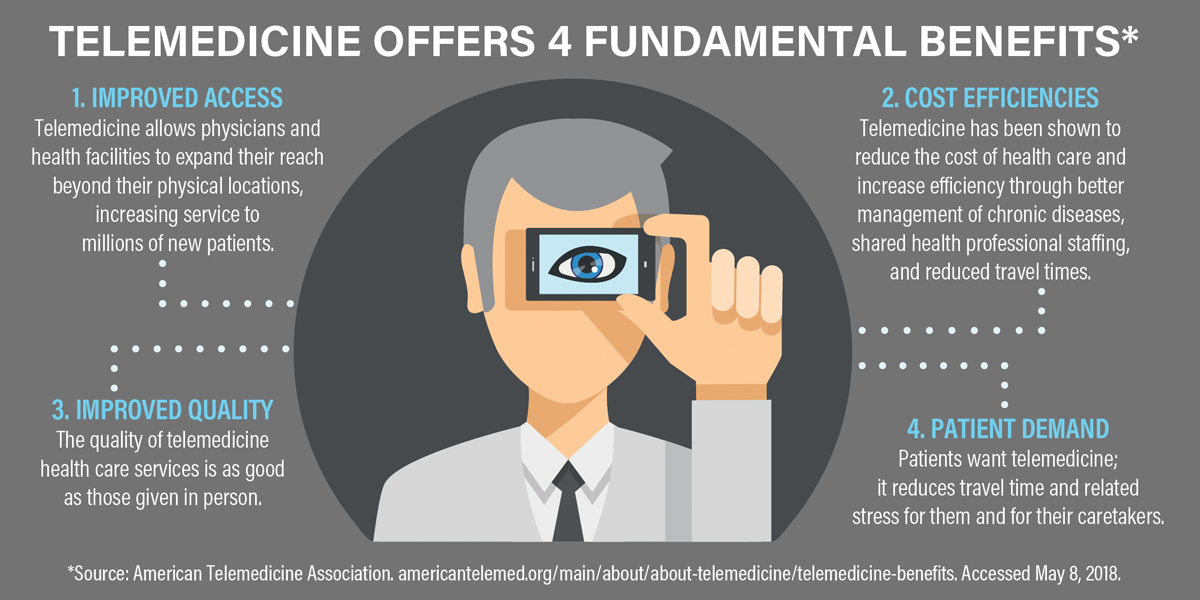
A TREND THAT DESERVES SUPPORT
Doctors can now remotely monitor cardiac rhythms, blood glucose, and many other metrics. As of 2017, more than 7 million patients were using remote monitoring and connected medical devices as an integral part of their care routines.4 The number of remotely monitored patients is estimated to reach 50.2 million by 2021.4 Let’s make sure our patients with AMD are among those reaping the benefits of remote monitoring technologies.
1. Chew EY, Clemons TE, Bressler SB, et al; for the AREDS2-HOME Study Research Group. Randomized trial of a home monitoring system for early detection of choroidal neovascularization home monitoring of the eye (HOME) study. Ophthalmology. 2014;121(2):535-544.
2. Chew EY, Clemons TE, Harrington M, et al; for the AREDS2-HOME Study Research Group. Effectiveness of different monitoring modalities in the detection of neovascular age-related macular degeneration: The Home Study, report number 3. Retina. 2016;36(8):1542-1547.
3. Chew EY, Clemons TE, Bressler SB, et al; for the AREDS2-HOME Study Research Group. Randomized trial of the ForeseeHome monitoring device for early detection of neovascular age-related macular degeneration. The HOme Monitoring of the Eye (HOME) study design – HOME Study report number 1. Contemp Clin Trials. 2014;37(2):294-300.
4. mHealth and Home Monitoring. Berg Insight. berginsight.com/ReportPDF/ProductSheet/bi-mhealth8-ps.pdf. Accessed May 4, 2018.






