

Glaucoma’s etiology is multifactorial and not fully understood. The only modifiable risk factor in glaucoma is intraocular pressure (IOP), and all current treatment approaches focus on lowering IOP.
IOP is determined by the ratio of aqueous humor production to aqueous outflow. The resistance to aqueous outflow through the conventional pathway of the trabecular meshwork (TM) is influenced by a second pressure worth understanding in glaucoma at the level of the episcleral veins: episcleral venous pressure (EVP) . With an understanding of only one of the pressures involved in glaucomatous pathophysiology, clinicians have only one part of the story.
EVP
The first key in comprehending EVP’s role in glaucoma is to understand the anatomic pathway of aqueous outflow. In the conventional pathway, aqueous flows through the TM into the canal of Schlemm. From Schlemm canal, aqueous enters into one of approximately 30 aqueous collector channels and then into the aqueous veins and episcleral veins. From there, aqueous is carried by the superior and inferior ophthalmic veins to the cavernous sinus, which joins with the systemic circulation.
When aqueous comes into contact with the episcleral veins, the result is a retrograde pressure that can slow aqueous drainage, which is what we call EVP. Normal EVP is reported to range between 8 mm Hg and 10 mm Hg.1-3 Measuring EVP is currently very difficult, and measurements are variable. Unlocking an accurate protocol to measure EVP would provide further clarity in our efforts to regulate aqueous outflow and manage IOP in patients with glaucoma.
According to the Goldmann equation (Figure), EVP is always additive to IOP, increasing the measured value.4 A more reliable and easily obtained method of measuring EVP would help providers predict and possibly alter the Goldmann equation.
There is currently no consistent and accurate measurement for EVP. Invasive and noninvasive measurement techniques have been explored; invasive techniques tend to be more accurate, but clinicians and patients naturally favor noninvasive methods.
Aihara et al described an indirect measurement technique to estimate EVP. In this approach, the clinician observes the anterior chamber angle while lowering the anterior chamber pressure until blood refluxes back into the collector channels and Schlemm canal (Video). This subjectively establishes the retrograde pressure and theoretical EVP.5,6 Other noninvasive techniques rely on observing venous compression. The examiner identifies an episcleral vein and gradually applies force to collapse the vein and determine the theoretical EVP.7,8
TREATMENT PATHWAYS
The importance of understanding an individual patient’s EVP is that it allows a more tailored treatment approach. IOP is the only modifiable risk factor at present. If we were able to more easily and accurately identify EVP, we would have another variable that could be modified to lower IOP either pharmacologically or surgically. For example, in a patient with Sturge-Weber syndrome or idiopathically elevated EVP, we could choose between a treatment to lower the EVP or one that uses an alternative pathway to avoid elevated EVP.
The pharmaceutical agents approved for treatment of glaucoma by the US Food and Drug Administration (FDA) that avoid the conventional outflow pathway aim instead at one of two alternative mechanisms: increasing uveoscleral outflow or decreasing aqueous production. In the glaucoma pipeline, latanoprostene bunod 0.024% (Vyzulta, Bausch + Lomb), netarsudil 0.02% (Rhopressa, Aerie Pharmaceuticals), and netarsudil 0.02%/latanoprost 0.005% (Roclatan, Aerie Pharmaceuticals) all have mixed mechanisms of action that include increasing TM outflow.9-11 The bonus here is that each of these drops is intended to lower EVP as well.12-15
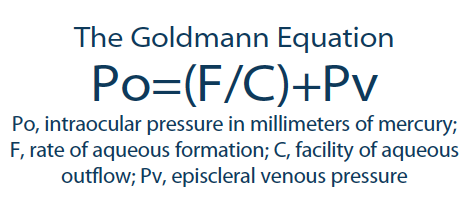
Figure. The Goldman equation dictates that IOP equals the sum of EVP and the quotient of the rate of aqueous formation divided by the facility of aqueous ouflow.
If EVP can be measured accurately in every patient, then these pipeline options may be more beneficially deployed—either as standalone treatments or as part of a drop cocktail—in patients with high EVP. Additionally, topical drops with the potential to lower EVP could be combined with TM-based minimally invasive glaucoma surgery (MIGS) procedures. If the target IOP is not reached postoperatively, an added drop may help anatomic outflow and enhance the MIGS mechanism.
MIGS AND EVP
MIGS procedures have rapidly become an important subset of glaucoma treatments, with positive momentum and expanding options. Among MIGS procedures that boost aqueous outflow, current FDA-approved models target outflow through the TM and Schlemm canal, the suprachoroidal or supraciliary space, and the subconjunctival space. Whether the surgery involves stenting, cutting, ablating, or dilating, the TM outflow pathway will always be affected by EVP.
It is important to remember that, using MIGS through these pathways, it is difficult to achieve IOPs below a normal physiologic EVP (7-8 mm Hg). This can be viewed as a positive characteristic because EVP can serve as a physiologic backstop against hypotony when a TM-based surgery is performed correctly. Having an accurate measurement of each patient’s EVP preoperatively would allow clinicians to take a truly individualized approach to MIGS. For example, if the patient’s EVP is high, then electing to pursue the suprachoroidal or supraciliary space for mild to moderate glaucoma and the subconjunctival space for moderate to severe glaucoma may be the best plan. If EVP is low, utilizing the TM-Schlemm canal outflow pathway might provide high effectiveness with minimal hypotony risk.
CONCLUSION
The glaucoma puzzle remains unfinished, but realizing the role that EVP plays in aqueous outflow can be helpful in determining optimal clinical treatment approaches. EVP measurement is not widely performed or considered at this time, but researchers, clinicians, and industry leaders continue to search for a better way to measure it.
- Sultan M, Blondeau P. Episcleral venous pressure in younger and older subjects in the sitting and supine positions. J Glaucoma. 2003;12(4):370-373.
- Toris CB, Yablonski ME, Wang YL, Camras CB. Aqueous humor dynamics in the aging human eye. Am J Ophthalmol. 1999;127(4):407-412.
- Zeimer RC, Gieser DK, Wilensky JT, et al. A practical venomanometer. Measurement of episcleral venous pressure and assessment of the normal range. Arch Ophthalmol. 1983;101(9):1447-1449.
- American Academy of Ophthalmology. Basic and Clinical Science Course Section 10: Glaucoma. San Francisco: American Academy of Ophthalmology, 2008.
- Aihara M, Lindsey JD, Weinreb RN. Reduction of intraocular pressure in mouse eyes treated with latanoprost. InvestOphthalmol Vis Sci. 2002;43:146-150.
- Aihara M, Lindsey JD, Weinreb RN. Episcleral venous pressure of mouse eye and effect of body position. Curr Eye Res. 2003;27(6):355-362.
- Sit AJ, Ekdawi NS, Malihi M, McLaren JW. A novel method for computerized measurement of episcleral venous pressure in humans. Exp Eye Res. 2011;92(6):537-544.
- Sit AJ, McLaren JW. Measurement of episcleral venous pressure. Exp Eye Res. 2011;93(3):291-298.
- Weinreb RN, Ong T, Scassellati Sforzolini B, et al. A randomized, controlled comparison of latanoprostene bunod and latanoprost 0.005% in the treatment of ocular hypertension and open angle glaucoma: The VOYAGER study. Br J Ophthalmol. 2015;99(6):738-745.
- Aerie Pharmaceuticals Rhopressa Rocket 4 Phase 3 6-Month Topline Results Conference Call. Aerie Pharmaceuticals. April 12, 2017. http://investors.aeriepharma.com/events.cfm. Accessed August 3, 2017
- Aerie Pharmaceuticals Roclatan Mercury 1 Phase 3 Topline Safety Results Conference Call. Aerie Pharmaceuticals. July19, 2017. http://investors.aeriepharma.com/events.cfm. Accessed August 3, 2017.
- Garcia GA, Ngai P, Mosaed S, and Lin KY. Critical evaluation of latanoprostene bunod in the treatment of glaucoma. Clin Ophthalmol. 2016;10:2035–2050.
- Dalton M. Rho-kinase inhibitors offer promise in glaucoma treatment. Eye World. March 2015. Accessed August 21, 2017.
- Brennan K. Lowering IOP: Will First-Line Options Change? Review of Ophthalmology. June 9, 2017 Accessed August 21, 2017.
- New glaucoma medications relax trabecular meshwork, lower episcleral venous pressure. Healio: Ocular Surgery News. October 2014. Accessed August 21, 2017.

