

A recent blossoming of technologies has increased the variety of microinvasive glaucoma surgery (MIGS) devices available for use in patients with mild to moderate glaucoma. Using an ab interno approach, MIGS procedures take advantage of most ophthalmologists’ familiarity with clear corneal incisions, avoid the need for conjunctival dissection, and result in minimal collateral tissue disruption.1
The use of MIGS is no longer a novelty or mystery, and optometrists interested in treating glaucoma to their full scope of practice should be well versed in the functionality and mechanisms of the various implants available. This will allow them to work with surgeons to provide the best possible solutions for their patients with glaucoma.
The management of glaucoma by optometrists is centered on the control of intraocular pressure (IOP). Appropriate use of topical antiglaucomatous medications has been shown to slow visual field loss in more than 90% of patients.2,3 Despite this evidence of success, nearly 65% of patients do not fully comply with their topical medication regimens.2,4
When surgery becomes necessary, procedures such as traditional trabeculectomy with bleb formation and implantation of intraocular drainage devices have demonstrated the ability to reduce IOP significantly, but they are also accompanied by increased complication rates, substantial failure rates, and inconsistent results.5
With these factors in mind, MIGS has the opportunity to make a swift and significant clinical impact in the care of patients with glaucoma. At the time of this writing, four MIGS devices have received regulatory approval from the US Food and Drug Administration (FDA), and a fifth approval is pending. In addition, four devices have been awarded a US investigational device exception (IDE) and are undergoing clinical evaluation. These devices achieve IOP lowering through a variety of mechanisms of action (see Types of MIGS Devices and Regulatory Status below). This article reviews the available and investigational devices and explains the ways they take advantage of the eye’s anatomy to help manage IOP.
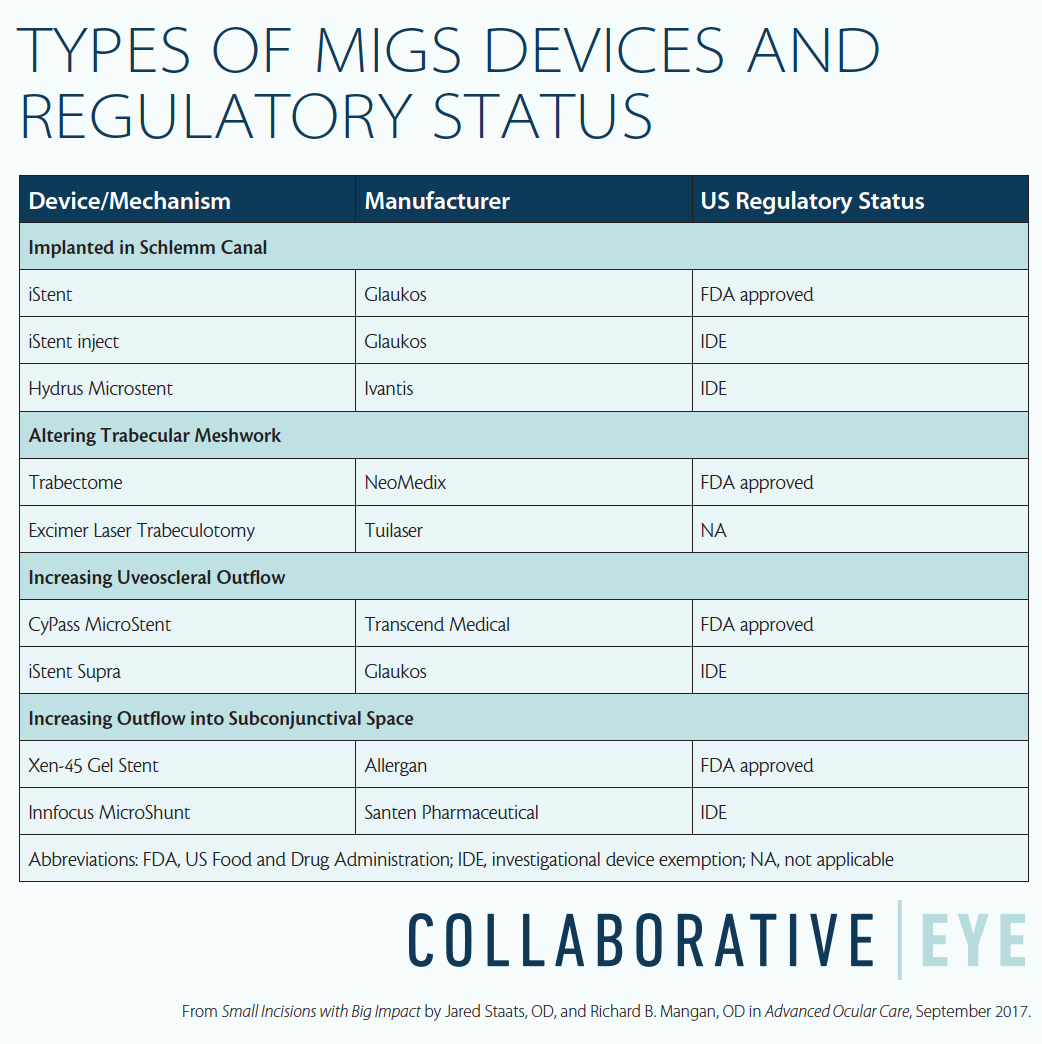
INCREASING CONVENTIONAL TRABECULAR OUTFLOW
The original MIGS devices reduced the resistance of the trabecular meshwork to aqueous outflow by allowing direct communication of aqueous between the anterior chamber and the canal of Schlemm. Although the efficacy of these devices is limited by episcleral venous pressure, their safety profiles are exceptional, making them an appealing treatment for patients with mild to moderate glaucoma.1
The iStent (Glaukos) is the smallest implantable device approved by the FDA and is among the most popular and well-researched MIGS devices. The iStent is positioned directly into Schlemm canal via the trabecular meshwork in the anterior chamber angle, using a preloaded single-use inserter.
A newer generation device, the iStent inject (Glaukos), is undergoing clinical evaluation. The iStent inject varies only slightly in size, manufacturing, and material from the original iStent. Its greatest potential benefit is the ability to implant two devices using a single injector without exiting the globe.
The Hydrus Microstent (Ivantis) is also implanted into Schlemm canal, but it is nearly 25 times than the iStent. The Hydrus is curved and is large enough to dilate 90° of the canal, permitting direct fluid exchange with the anterior chamber aqueous over a larger surface area than the iStent.
The efficacy and safety of the iStent and Hydrus devices have been evaluated as standalone interventions and in combination with cataract extraction.6,7 (In the case of the iStent, standalone procedures could utilize one, two, or three devices, and in combination with cataract surgery one or two devices.6) Reduction in IOP was comparable between the two devices, loosely averaging 2 to 4 mm Hg reduction in comparison to medicated preoperative baseline.6,7 Multiple iStents had an additive benefit in comparison with single implants in both standalone and combined procedures.6 In addition to reduction in IOP, significant reduction in dependence on topical hypotensive medications was observed following both iStent and Hydrus implantation.6,7 The benefits are especially positive when viewed in relation to risk, with a safety profile mirroring that of cataract extraction alone. Revision rate is as low as 1 to 2%.1
Two larger studies evaluated a single iStent implantation in combination with cataract extraction.8,9 These studies consistently found modest reduction in IOP (1.5–2.0 mm Hg; 8–15% reduction) compared with medicated baseline. Although the IOP decreases were modest, they resulted in significant reduction in dependence on topical medications and improved long-term IOP findings at 2-year follow-up.8,9
Currently, iStent use is largely restricted to use in combination with cataract extraction due to FDA regulations and insurance reimbursement.
ALTERING THE TRABECULAR MESHWORK
The Trabectome (Neomedix) is representative of devices capable of performing ab interno trabeculotomy. In this technique, focal energy from an electrode tip is used to ablate 30° of the nasal trabecular meshwork, providing aqueous access to Schlemm canal.1,10 Another approach to altering the trabecular meshwork is used in excimer laser trabeculotomy (ELT), in which an excimer laser (Aida; Tuilaser) creates small, precise perforations of the trabecular meshwork without induced scarring. This procedure achieves reduction in resistance to aqueous outflow similar to that of electrosurgical ab interno trabeculotomy.11 Either can be performed in combination with cataract extraction or as a standalone procedure. Postoperative IOP reduction with these devices ranged from 4 to 10 mm Hg in comparison with unmedicated baseline IOP, with an associated decrease in dependence on hypotensive medications.10,11
A study comparing the Trabectome combined with cataract extraction and two iStent implants combined with cataract extraction found similar IOP lowering effects and safety profiles for the two MIGS procedures.12 A study including patients with pseudoexfoliative glaucoma showed a particularly strong response to treatment.13
The safety profiles of these procedures are equally encouraging. After ab interno trabeculectomy, a transient intraoperative blood reflux can be expected, typically self-limited and self-resolving.10 More significant complications occur rarely in the form of synechiae formation, although these are less common than comparable complications in tradition glaucoma surgeries.10-12
INCREASING UVEOSCLERAL OUTFLOW
Recently introduced MIGS devices have begun utilizing the suprachoroidal space, a potential space that offers unobstructed aqueous outflow. This increases the opportunity for more efficacious IOP reduction. The Cypass MicroStent (Transcend Medical) is a flexible, perforated stent that conforms to the curvature of the sclera and is surgically guided into the supraciliary space. The COMPASS II study showed a mean 7.5 mm Hg decrease in IOP from preoperative washout baseline, as well as reduced dependence on topical hypotensive medications at 2-year follow-up.14
The iStent Supra is a similar device, with clinical evaluation getting under way only recently.1
As with traditional penetrating glaucoma surgeries, taking advantage of this potential space leaves the patient susceptible to IOP fluctuation in the postoperative period.14,15 Due to the anatomic outflow route, the anterior chamber will maintain its shape despite hypotony. Hypertensive changes can be more concerning, as the procedure does not permit acute relief techniques, and scar tissue is not preventable or reversible.15
PERMITTING OUTFLOW INTO SUBCONJUNCTIVAL SPACE
Conventional glaucoma surgical techniques such as trabeculectomy and tube-shunt implants allow aqueous to escape into the potential space under the Tenon capsule.1 Similar to their conventional counterparts, MIGS devices that utilize the subconjunctival space are not dependent on episcleral venous pressure and are susceptible to localized scarring.
Similar to other MIGS devices, the Xen-45 Gel Stent (Allergan) is implanted using a preloaded injector. After its injection into the subconjunctival space, bleb formation has been observed in both standalone and combined cataract procedures.1 In a recent study, mean IOP was reduced from a preoperative medicated 21.2 mm Hg to 15.0 mm Hg postoperatively, and medications were reduced from a mean of 3.00 preopreratively to 0.17 at 1-year follow-up. Of patients who completed the study, 90% had IOPs of less than 18.0 mm Hg with no medication.16
In comparison with conventional glaucoma surgeries, the safety profile and success rate with the Xen implant is promising. Postoperative bleb encapsulation was observed in one eye (3.3%) by 1 year.16Additionally, the implant design has an intrinsic outflow resistance of 6 to 8 mm Hg, reducing the likelihood of hypotony.17 Despite these strong early findings, further significant publications are lacking.
Recently, the FDA classified the Innfocus MicroShunt (Santen Pharmaceutical) as a MIGS device, even though the implantation process requires an ab externo approach with conjunctival dissection.1 An early trial reported remarkable reductions in IOP and medication use, more substantial than those of any of the other MIGS techniques mentioned above.18
CONCLUSION
Optometrists are well positioned to offer new MIGS therapeutic alternatives to their patients with glaucoma. These procedures can provide significant reductions in IOP, increased independence from topical medications, and greatly improved safety profiles compared with penetrating glaucoma procedures. These surgical advances offer exciting collaborative management opportunities for optometrists interested in being involved at the forefront of glaucoma intervention.
MIGS devices such as the iStent, which create direct communication between the anterior chamber and Schlemm canal, are well researched and widely available. The iStent implant provides modest reduction from baseline medicated IOP when combined with cataract extraction, and the iStent inject, if approved, will give surgeons the capability to swiftly and safely introduce multiple implants into the anterior chamber angle. Other implants, and procedures such as ab interno trabeculotomy, provide alternatives to the iStent with larger areas of aqueous communication.
Devices that permit filtration to the suprachoriodal or subconjunctival space hold the potential for greater IOP reductions. The CyPass MicroStent, Xen-45 Gel Stent, and Innfocus MicroShunt have shown the potential to reduce IOP to levels beyond those achieved by more established MIGS devices. While the Schlemm canal devices boast a comparatively better safety profile, the suprachoroidal and subconjuctival space implants have demonstrated higher success rates than conventional glaucoma surgeries with comparable mechanisms.
Unfortunately, there is little research regarding the long-term efficacy of MIGS devices. With more randomized trials and comparative studies on the horizon, a growing understanding of the indications for the individual MIGS procedures will become more apparent. Getting to know these devices—their capabilities, indications, contraindications, and potential risks and benefits—will allow optometrists to better participate in the surgical management of their patients with glaucoma.
- Chen DZ, Sng CCA. Safety and efficacy of microinvasive glaucoma surgery. J Ophthalmol. 2017;2017:3182935. doi: 10.1155/2017/3182935.
- Bilger M, Wong TT, Howard KL, et al. Study on Incentives for Glaucoma Medication Adherence (SIGMA): study protocol for a randomized controlled trial to increase glaucoma medication adherence using value pricing. Trials. 2016;17(1):316.
- Nordstrom BL, Friedman DS, Mozaffari E, Quigley HA, Walker AM. Persistence and adherence with topical glaucoma therapy. Am J Ophthalmol. 2005;140(4):598-606.
- van Dulmen S, Sluijs E, van Dijk L, de Ridder D, Heerdink R, Bensing J. Patient adherence to medical treatment: a review of reviews. BMC Health Serv Res. 2007;7:55.
- Gedde SJ, Schiffman JC, Feuer WJ, Herndon LW, Brandt JD, Budenz DL; Tube Versus Trabeculectomy Study Group. Three-year follow-up of the tube versus trabeculectomy study. Am J Ophthalmol. 2009;148(5):670-684.
- Katz LJ, Erb C, Carceller GA, et al. Prospective, randomized study of one, two, or three trabecular bypass stents in open-angle glaucoma subjects on topical hypotensive medication. Clin Ophthalmol. 2015;9:2313-2320.
- Pfeiffer N, Garcia-Feijoo J, Martinez-de-la-Casa JM, et al. A randomized trial of a Schlemm’s canal microstent with phacoemulsification for reducing intraocular pressure in open-angle glaucoma. Ophthalmology. 2015;122(7):1283-1293.
- Craven ER, Katz LJ, Wells JM, Giamporcaro JE. Cataract surgery with trabecular micro-bypass stent implantation in patients with mild-to-moderate open-angle glaucoma and cataract: two-year follow-up. J Cataract Refract Surg. 2012;38(8):1339-1345.
- Samuelson TW, Katz LJ, Wells JM, Duh YJ, Giamporcaro JE. Randomized evaluation of the trabecular micro-bypass stent with phacoemulsification in patients with glaucoma and cataract. Ophthalmology. 2011;118(3):459-467.
- Minckler DS, Baerveldt G, Alfaro MR, Francis BA. Clinical results with the Trabectome for treatment of open-angle glaucoma. Ophthalmology. 2005;112(6):962-967.
- Wilmsmeyer S, Philippin H, Funk J. Excimer laser trabeculotomy: a new, minimally invasive procedure for patients with glaucoma. Graefes Arch Clin Exp Ophthalmol. 2006;244(6):670-676.
- Gonnermann J, Bertelmann E, Pahlitzsch M, Maier-Wenzel AB, Torun N, Klamann MK. Contralateral eye comparison study in MICS & MIGS: Trabectome vs. iStent inject. Graefes Arch Clin Exp Ophthalmol. 2017;255(2):359-365.
- Ting JL, Damji KF, Stiles MC. Ab interno trabeculectomy: outcomes in exfoliation versus primary open-angle glaucoma. J Cataract Refract Surg. 2012;38(2):315-323.
- Vold S, Ahmed II, Craven ER, et al; CyPass Study Group. Two-year COMPASS trial results: supraciliary microstenting with phacoemulsification in patients with open-angle glaucoma and cataracts. Ophthalmology. 2016;123(10):2103-2112.
- Hoeh H, Vold SD, Ahmed IK, et al. Initial clinical experience with the CyPass Micro-Stent: safety and surgical outcomes of a novel supraciliary microstent. J Glaucoma. 2016;25(1):106-112.
- Pérez-Torregrosa VT, Olate-Pérez Á, Cerdà-Ibáñez M, et al. Combined phacoemulsification and Xen45 surgery from a temporal approach and 2 incisions [article in English, Spanish]. Arch Soc Esp Oftalmol. 2016;91(9):415-421.
- Lewis RA. Ab interno approach to the subconjunctival space using a collagen glaucoma stent. J Cataract Refract Surg. 2014;40(8):1301-1306.
- Batlle JF, Fantes F, Riss I, et al. Three-year follow-up of a novel aqueous humor microshunt. J Glaucoma. 2016;25(2):e58-65.

