
Most eye care practitioners know that dry eye disease (DED) and ocular allergy often overlap and that each entity can trigger symptoms that can affect patients’ quality of life. These conditions are also frequently concomitant. Because DED and allergy are each identified by their symptoms and because they share similar symptom profiles, however, making a correct diagnosis can often be challenging. One potential consequence is that directing appropriate therapy can be complicated, but recent evidence suggests that differentiating between the two clinical entities is nevertheless important.
Studies looking at meibomian gland morphology in individuals with chronic allergic conjunctivitis indicate that long-standing allergy may predispose the eye to tissue changes that eventually lead to the development of DED.1 Although eye care providers have suspected that certain manifestations of DED might increase the intensity of the allergic response, it now seems that the risk profile can function in the other direction as well.
THE PHYSIOLOGY OF OCULAR ALLERGY
To properly appreciate the interaction between DED and ocular allergy, it is important to recognize the physiology of the latter.
Clinically, ocular allergy is a type 1 hypersensitivity reaction in a sensitized individual that is defined by adjacent immunoglobulin E molecules crosslinking with an antigen. This interaction triggers local mast cell degranulation, in turn setting off the release of biochemical mediators involved in the inflammatory cascade—most notably, histamine. The fact that this is a cascade event has several implications. Ocular allergy tends to worsen progressively in proportion to the duration of exposure to the particular antigen. If left untreated, the allergic response has the potential to intensify over time and lead to tissue damage that predisposes the sufferer to developing other ocular surface disease(s). As with any cascade event, removing the instigating entity or stopping the series of events at any stage prevents the downstream consequences.
The physiology of ocular allergy explains why antigen avoidance and dual-acting antihistamines–mast cell stabilizers are mainstays of treatment. There are now several effective ocular allergy agents available on the market: alcaftadine (Lastacaft; Allergan), olopatadine HCl (Pataday 0.2%, Novartis; Pazeo 0.7%, Novartis), and bepotastine besilate (Bepreve; Bausch + Lomb) are the best known. The goal with these agents is to suppress the inflammatory response in hopes of alleviating the accompanying symptoms.
The recent market availability of newer allergen testing kits has simplified providers’ ability to identify the offending allergen so that they can counsel patients on avoidance strategies. The practicalities of implementation may dictate whether practitioners find this advantageous. For a brief time, my colleagues and I offered a broad-panel allergen test to patients with suspected allergies, but the cost of the test and what it produced ultimately did not make sense. Although point-of-care testing was a consideration, we did not believe it would significantly affect our decision-making. As a result, we tend to treat what we see and refer patients for follow-up with their primary care doctor or for specific testing with an allergist.
ORAL AGENTS AND IMPLICATIONS FOR DED
Many patients with systemic allergies self-medicate with oral antihistamines, and their good intentions may lead to unwanted sequelae that have importance for the health of their eyes. The use of oral antihistamines for treating ocular allergy is controversial because there is not much evidence that their use has any benefit for the ocular manifestations. The use of oral antihistamines can, however, dry the cornea, which in turn intensifies the allergic response. In most cases, patients are unaware that the oral antihistamines they are using to relieve their primary allergy symptom are producing a deleterious effect on the ocular surface that worsens another component of their allergic response.
Given the popularity of over-the-counter options for treating systemic allergies, it is not at all uncommon for patients to experience eye dryness, which they may attribute to ocular allergies. Because of the crossover in symptoms between the two conditions (due in part to a shared physiology), it may be difficult for practitioners to parse out the specific etiology. As a matter of practicality, it then becomes incumbent on the eye care practitioner to talk with patients taking systemic allergy medications about the potential ocular side effects. It would also be wise to offer treatment, such as meibomian gland expression, if the agents are going to used, because any kind of instigator—be it decreased aqueous production or increased evaporative stress—leads to more progressive types of DED.
ALLERGY AS A POTENTIAL CONTRIBUTOR TO MEIBOMIAN GLAND REMODELING
Differentiating ocular allergy from DED and other ocular surface conditions is challenging, but emerging evidence seems to suggest that meibomian gland morphology may change as a potential consequence of chronic allergies, which in turn potentiates the eye toward DED (Figures 1 and 2).1 Longitudinal data are needed to support this theory.
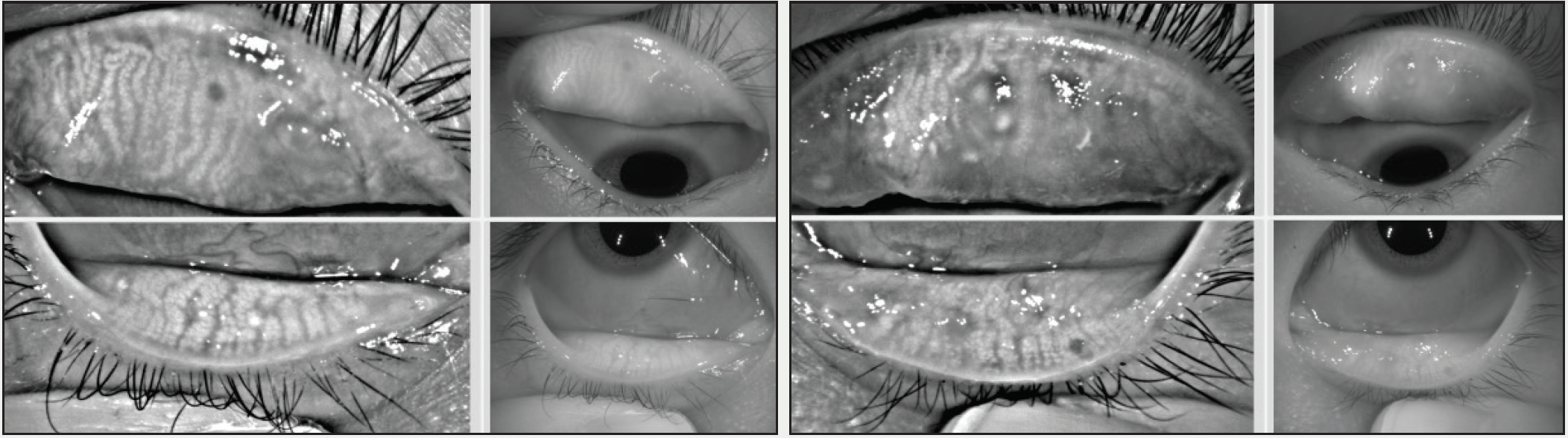
Figure 1. A 22-year-old Asian woman symptomatic for chronic allergy exhibits changes in meibomian gland morphology.
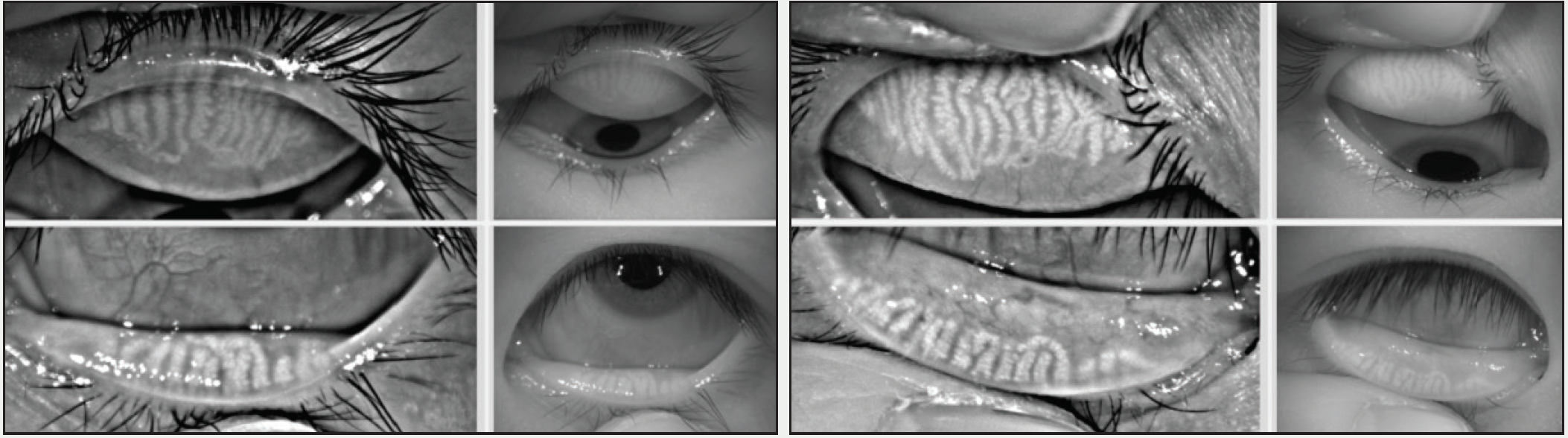
Figure 2. This 23-year-old Asian woman symptomatic for chronic allergy also exhibits changes in meibomian gland morphology.
Another aspect of the aforementioned study that bears further exploration is that gland tortuosity was identified in the meibomian glands of pediatric patients as young as 7 years of age with allergic conjunctivitis.1 Although it has long been suspected that diminished meibomian gland capacity and subsequent tear film dysfunction might predispose a person to an allergic response, this is yet another way to further examine the effects of long-term ocular allergy and its contribution to more severe allergy subtypes such as vernal keratoconjunctivitis and atopic keratoconjunctivitis.2
At this time, it is uncertain whether changes in the meibomian gland structure in young individuals are caused by the inflammation inherent to ocular allergy; whether they may be from mechanical eye rubbing, which then compressed and distorted the glands; or whether there may be a genetic predisposition toward morphologic changes in meibomian structure that supersedes the allergic cascade. There may also be a cause-and-trigger effect in which alterations to the gland reduced mucin production, and the resulting increase in the natural tear’s evaporative nature reduced the protective barrier, making it easier for antigens to pass through and cause additional damage.
Again, more in-depth testing and observation will be necessary to fully illustrate the relationship among allergy, DED, and meibomian gland structure and function. For now, these findings should encourage practitioners to identify severe allergy in young patients and treat it aggressively to thwart potential alterations to the meibomian glands that could have long-term consequences.
CONCLUSION
Both DED and ocular allergy are symptom-based clinical entities, but nuances in their presentations can help with differentiation. I tend to look for ocular allergy based on the presenting signs and symptoms, while recognizing that redness, irritation, and itching that are common to ocular allergy are also manifest in blepharitis, DED, and other ocular surface conditions. Thus, the initial presentation is a starting point to guide the ocular examination. One key to differentiating ocular allergy from blepharitis, for example, is to localize the presence of itching or irritation to the globe and the lids. In particular, there tends to be a stronger response on the upper lid in an eye with ocular allergy than with blepharitis.
Advanced diagnostics such as meibography have helped clinicians appreciate that architectural changes in gland structure once thought to be confined to the DED cascade may influence and be influenced by other ocular surface entities. This emerging evidence suggests that the crossover of DED and ocular allergy is not so simple. Although it is fairly common to see these entities in regular clinical practice, new understanding of how one may lead to the other and vice versa raises the stakes for proper recognition and appropriate treatment.
- Schachter S, Schachter A, James E, Hom MM. Allergic conjunctivitis and total symptom score. Invest Ophthalmol Vis Sci. 2016;57(12):5702.
- Fasanella V, Agnifili L, Mastropasqua R, et al. In vivo laser scanning confocal microscopy of human meibomian glands in aging and ocular surface diseases. Biomed Res Int. 2016;2016:7432131.

